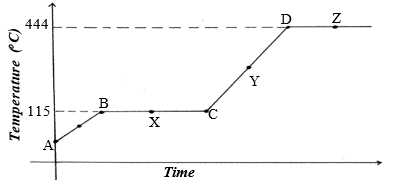
1. The diagram below shows the heating curve of a pure substance. Study it and answer the questions that follow:
(a) What physical changes are taking place at points X and Z?
(b)Explain what happens to the melting point of sodium chloride added to this substance
2. (a) State two differences between luminous flame and non-luminous flame
(b) It is advisable to set a Bunsen burner to luminous flame prior to an experiment. Explain
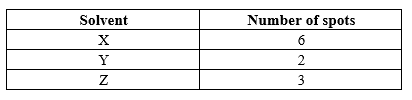
3. The paper chromatography of a plant extract gave the following results: (a) Which is the most suitable solvent for purifying the extract? Explain
(b) Ball pen cannot be used to mark solvent front in the above chromatography. Explain 4. Name the process which takes place when:
(a) Solid Carbon (Iv) Oxide (dry ice) changes directly into gas
(b) A red litmus paper turns white when dropped into chlorine water
(c) Propene gas molecules are converted into a giant molecule
5. A sample of copper turnings was found to be contaminated with copper (II) oxide. Describe how a sample of copper metal can be separated from the mixture
6. Copper (II) oxide and charcoal are black solids. How would you distinguish between the two solids?
7. a) What is chromatography?
b) Give two applications of chromatography
8. The two elements P and R were separately burned in air, the products gave the results recorded in the table below:
(a) Suggest the identity of element R. ……………………………………………..……..
(b) Describe how the nature of the solutions of the of the oxides were determined
9. The diagram below represents a paper chromatography for the three brands of soft drinks containing banned artificial food additives.
A and C found to contain the banned artificial food additives. Which numbers indicate the banned artificial food additives?
10. Without using any laboratory chemical, describe a simple laboratory experiment to distinguish between calcium hydrogen carbonate and sodium hydrogen carbonate
11. Substance Q has a melting point of 15oC and boiling point of 70oC.
(a) On the same axes, draw the melting point and boiling point graph for Q and the room temperature
(a) State the physical state of substance Q at room temperature 12. Cooking oils comprise of a mixture of compounds which have a boiling point range of 23oC to 27oC.
(i) What evidence is then to support the statement that cooking oil is a mixture?
(ii)Name another experimental technique that could be used to confirm your answer in part (i) above
13. A form 1 student carried out the separation as shown in the set-up below:-
i) Identify the method above.................................................................................
ii) Give one of its disadvantages
iii) Name a mixture which can be separated by the set-up above
14. What is meant by melting point and boiling point of a substance?
13. A form 1 student carried out the separation as shown in the set-up below:-
ii) Give one of its disadvantages
iii) Name a mixture which can be separated by the set-up above
14. What is meant by melting point and boiling point of a substance?
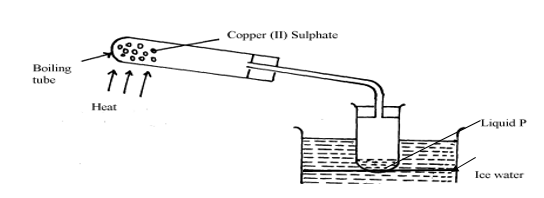
15. The apparatus below were used by a student to study the effect of heat on hydrated copper II sulphate
17. The diagram below shows chromatograms of blood samples obtained from three athletes. One athlete used illegal drug to improve performance in competition.
(a) Name the line marked M …………………………………………………. (b)Identify the athlete who used illegal drug ……………... ……………………….
18. Classify the following processes as chemical changes or physical changes
19. Give two reasons why a luminous flame is not used for heating purposes
20. Classify the following processes as chemical changes or physical changes
Process physical or chemical
Neutralization ………………………………………
Sublimation ………………………………………
Fractional distillation ………………………………………..
Displacement reaction …………………………………………
21. Give two reasons why a luminous flame is not used for heating purposes
22. State two criteria for determining the purity of a substance
23. Study the information in the table below and answer the questions.
Displacement reaction …………………………………………
21. Give two reasons why a luminous flame is not used for heating purposes
22. State two criteria for determining the purity of a substance
23. Study the information in the table below and answer the questions.
i) A mixture contains ethene, Hydrogen and ammonia gases. Explain how a sample of hydrogen gas can be obtained from this mixture.
24. a)i) The diagram below show spots of a pure substance A, B, and C on a chromatography paper. Spot D is that of a mixture
After development A, B, and C were found to have moved 8cm, 3cm and 6cm respectively. D had separated into two spots which had moved 6cm and 8cm. On the diagram above;
I. Label the baseline (origin)
II. Show the positions of all the spots after development
ii) Identify the substances present in mixture D
b) Describe how solid ammonium chloride can be separated from a solid mixture of ammonium chloride and anhydrous calcium chloride
c) The table below shows liquids that are miscible and those that are immiscible
Use the information given in the table to answer that questions that follow;
i) Name the method that can be used to separate L1 and L2 from a mixture of the two
ii) Describe how a mixture of L2 and L4 can be separated
25. A student left some crushed fruit mixture with water for some days. He found the mixture had fermented. He concluded that the mixture was contaminated with water and ethanol with boiling point of 100oC and 78oC respectively. The set-up of apparatus below are used to separate the mixture.
(i) Name the piece of apparatus labelled W 25. A student left some crushed fruit mixture with water for some days. He found the mixture had fermented. He concluded that the mixture was contaminated with water and ethanol with boiling point of 100oC and 78oC respectively. The set-up of apparatus below are used to separate the mixture.
(ii) What is the purpose of the thermometer in the set-up?
iii) At which end of the apparatus W should tap water be connected?……………………………
(iv) Which liquid was collected as the first distillate? Explain (v) What is the name given to the above method of separating mixture?
(vi) State two applications of the above method of separating mixtures
(vi) What properties of the mixture makes it possible for the component to be separated
by the above methods?
26. The set-up below was used to separate a mixture:-
(a) Name the apparatus missing in the set-up
(b) Give one example of mixture T
(c) What is the name of this method of separation
27. a) The diagram below shows a set – up used by a student to find out what happens when Copper (II) sulphate crystals are heated.
(i) State the observations made when the blue copper (II) sulphate crystals are heated.
(ii) Identify liquid Y and write an equation for its formation.
b) Pellets of sodium hydrogen and anhydrous Copper (II) sulphate were put in separate Petri- dishes and left in the open for two hours. Explain the observation in each Petri-dish.
28. The chromatography below shows the constituents of a flower extract using an organic solvent:-
(a) (i) Name a possible organic solvent you can use for this experiment
(ii) State one property that makes the red pigment to move the furthest distance from M
(iii) Describe how one could get a sample of yellow pigment
(iv) On the diagram indicate solvent front
(b) Describe how Aluminium chloride can be separated from a mixture of aluminium chloride
and sodium chloride
29. Study the information below and answer the questions that follow:
Describe how the mixture of solid R, S, and V can be separated
30. Given a mixture of lead (II) oxide, ammonium chloride and sodium chloride, describe how this mixture can be separated to obtain a sample of each.
31. The setup below was used to separate two miscible liquids Q and T (Boling points; Q =98° C, T=78°C)
(b)Identify Distillate X
32. Name the process which takes place when:
(a) Solid Carbon (IV) oxide (dry ice) changes directly into gas.
(b) A red litmus paper turns white when dropped into chlorine water.
(c) Propene gas molecules are converted into a giant molecule.
33. The following diagram shows a paper chromatogram of substances A, B, C, and D which are coloured
(a) Indicate the solvent front on the chromatogram
(b)Which substance is pure? ………………………………………..
(c) Substance E is a mixture of C and D. Indicate its chromatogram in the diagram
34. Study the information below and answer the following questions. A mixture contains three solids A, B, and C. The solubility of these solids in different liquids is as shown below:-
Explain how you will obtain sample C from the mixture
35. State and explain the observations made when iodine crystals is heated in a boiling tube?
35. State and explain the observations made when iodine crystals is heated in a boiling tube?

























Iam I able to get the answers of THE questions
ReplyDeleteNagaland Board 10th Model Question Paper 2022 Blueprint Available Students you can Download in Pdf format form this web page below given links. HSLC Regular and Private Students Prepare their Exam from these Sample Paper, Students have to Register for Appearing in Public Exam 2022. NBSE 10th Model Paper 2022 Provide SSLC Latest and Last Year Exam Study Material for Syllabus, Question Paper etc, Hindi, English Medium Pdf Format. So if you are also among those students who have Registered them self as a Regular or Private Student are Suggested to Download these Nagaland Board HSLC Sample Paper 2022 must Prepare all Subjects.
ReplyDeleteWhere are the answers please
ReplyDelete