1. Study the flow chart below and answer the questions that follow:
a) Name reagent Z.
b) Describe the process which takes place in step 2.
c) Identify the white solid.
2. a) Starting from solid magnesium oxide, describe how a solid sample of magnesium hydroxide
can be prepared.
b) Give one use of magnesium hydroxide.
3. Starting with lead (II) oxide, describe how you would prepare a solid sample of
lead (II) Carbonate
4. Study the diagram below and answer the questions that follow:
(a) Name the two salts formed in tube A
(b) State the observations made in tube C
(c) Name gas P
5. Study the information in the table below and answer the questions that follow:-
(a) Complete the table by filling in the blank spaces (i) , (ii) (iii), and (iv)
(b) Identify the particles which are electrically charged
6. Sodium Carbonate Decahydrate crystals were left exposed on a watch glass for two days.
a) State the observations made on the crystals after two days.
b) Name the property of salts investigated in the above experiment
7. Starting with sodium oxide, describe how a sample of crystals of sodium hydrogen carbonate
may be prepared
8. In an experiment, ammonium chloride was heated in test-tube. A moist red litmus paper
placed at the mouth of test first changed blue then red. Explain these observations:-
9. Using dots (•) and cross (x), show the structure of ammonium ion
10. a) Give the name of each of the processes described below which takes place when salts are
exposed to air for sometime
i) Anhydrous copper sulphate becomes wet
ii) Magnesium chloride forms an aqueous solution
iii) Fresh crystals of sodium carbonate, Na2CO3.10H2O become covered with white powder
of formula Na2CO3.H2O
b) Write the formula of the complex ion formed in each of the following reactions described
below;
i) Zinc metal dissolves in hot alkaline solution
ii) Copper hydroxide dissolves excess ammonia solution
11 (a) Write an equation to show the effect of heat on the nitrate of:-
(i) Potassium
(ii) Silver
12. (a) The scheme below shows some reactions starting with magnesium oxide. Study it and
answer the questions that follow:-
(i) Name the reagents used in steps 2 and 4
(ii) Write an equation for the reaction in step 3
(iii) Describe how a solid sample of anhydrous magnesium carbonate is obtained in step 5
13. In the preparation of magnesium carbonate, magnesium was burnt in air and the product
collected. Dilute sulphuric acid was then added and the mixture filtered and cooled. Sodium
carbonate was added to the filtrate and the contents filtered. The residue was then washed and
dried to give a white powder.
(a) Give the name of the product
(b) Write the chemical equation for the formation of the product
(c) (i) Name the filtrate collected after sodium carbonate was added.
(ii) Write down the chemical formula of the white powder
(d) Write a chemical equation for the reaction between product in (a) and the acid
(e) Write an ionic equation to show the formation of the white powder.
(f) Write an equation to show what happens when the white powder is strongly heated.
(g) Identify the ions present in the filtrate after addition of sodium carbonate.
(h) What is the name given to the reaction that takes place when sodium carbonate was
added to the filtrate?
(i)Explain the observations made when crystals of sodium carbonate decahydrate are left
exposed to the atmosphere for two days
14. a) Give the name of each of the processes described below which takes place when salts are
exposed to air for sometime
i) Anhydrous copper sulphate becomes wet
ii) Magnesium chloride forms an aqueous solution
iii) Fresh crystals of sodium carbonate, Na2CO3.10H2O become covered with white powder
of formula Na2CO3.H2O
15. You are provided with the following:- solid lead (II) nitrate, magnesium oxide powder,
dilute sulphuric (VI)acid and distilled water. Describe how you can prepare a dry sample
of lead (II) sulphate
16. Use the scheme to answer the questions that follow:
(a) Identify solid N ……………………………………………………………….
(b) Write a balanced equation for the formation of Q
(c) Write the formula of the complex ion formed when sodium hydroxide is added to
solution L in excess
17. When exposed to air, crystals of hydrated sodium carbonate loses water of crystallizations;-
(i) Name this process
(ii) Write the formula of hydrated sodium carbonate
18. A student poured sodium iodide solution into a small portion of solution Q, a yellow
precipitate was formed.
(i) Which ion was most likely in solution Q?
(ii) Write an ionic equation leading to the formation of the yellow precipitate
19. Calcium oxide can be used as a solid drying agent for some laboratory gases. Explain
20. A piece of marble chips was strongly heated in air for about 30 minutes. Some drops of water were added drop by drop to the product when it was still warm.
Using equation, explain:
(i) What happens when the piece of marble chips is heated?
(ii) The reaction that takes place when water is added to the final warm product.
21. Study the flow chart below and answer the questions that follow
a) Identify ;
i) gases C and B
ii) Ions likely to be presented in solid A
22. Potassium nitrate crystals in a test-tube were heated strongly for some time. State the
observation made:
(a) When a glowing splint is introduced into the test-tube during the heating
(b) At the end of the heating
23. Name the process which takes place when:
(a) Anhydrous iron (III) chloride absorb water vapour from the air to form solution
(b) Zinc chloride vapour changes directly to zinc chloride solid
24. (a) Starting form solid magnesium oxide, describe how a solid sample of magnesium
hydroxide can be prepared
(b) Give one use of magnesium hydroxide
25. The diagram below represents a set-up that was used to show that part of air s used during burning
(a) State two sources of errors in this experiment
26. In an experiment the following solids were provided to form three students; Ca(NO3)2(s),
NaH2PO4(s); Mg(OH)Cl(s) and Fe(NH4)2(SO4)2. 6H2O. They were then told to dissolve the
given solids in differently in 20ml of water.
(a) Classify the given salts accordingly
(b) (i) Explain the process which takes place when FeCl3 is dissolved in water
(ii) A student placed a moist litmus paper on the product in (i) above. State and explain the
observation made
Sunday, 24 November 2019
Subscribe to:
Post Comments (Atom)




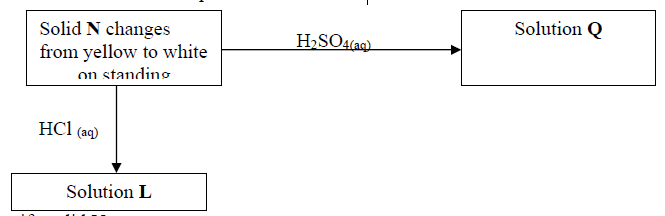











Answers
ReplyDeleteAm very thankful to you guys.
DeleteAnswer
Delete