1. Sulphur is extracted from underground deposits by a process in which three concentric pipes are
sunk down to the deposits as shown below
(a) Name the process represented above
(b) What is passed down through pipe J?
(c) Name the two allotropes of sulphur
2. Commercial sulphuric acid has a density of 1.8gcm3.
(a) Calculate the molarity of this acid
(b) Determine the volume of commercial acid in (a) above that can be used to prepare
500cm3 of 0.2M H2SO4 solution
3. Oleum (H2S2O7) is an intermediate product in the industrial manufacture of sulphuric acid
(a) How is oleum converted into sulphuric (IV) acid?
(b) Give one use of sulphuric acid
4. Differentiate between the bleaching action of chloride and sulphur (IV) oxide gas.
5. (i) Is concentrated sulphuric acid a weak acid or a strong acid?
(ii) Explain your answer in (i) above.
6. In the manufacture of sulphuric acid, sulphur (IV) oxide is oxidized to sulphur (VI) oxide.
a) Name the catalyst used
b) Write the equation representing the conversion of sulphur (IV) oxide to sulphur(VI)oxide
c) Explain using equations how dilute sulphuric acid is finally obtained from sulphur (VI) oxide
7. When a mixture of concentrated sulphuric acid and copper turnings is strongly heated,
a colourless gas and solid mixture of white and black solids are formed. When this solid
mixture is treated with distilled water, and filtered, a blue solution and black solid residue
are collected. Explain the observations on the solid mixture formed in the above experiment
8. The set-up below is used to prepare dry sulphur (IV) Oxide in the laboratory. Answer questions
that follow:
(a) Identify the mistake in the set-up
(b) Write an equation for the reaction in the set-up
(c) State how the polluting effects of the gas on the environment can be controlled
9. (a) State the observation made at the end of the experiment when a mixture of iron
powder and sulphur are heated in a test-tube
(b) Write an equation for the reaction between the product in (a) above and dilute
hydrochloric acid
(c) When a mixture of iron powder and sulphur is heated it glows more brightly than
that of iron fillings and sulphur. Explain this observation
10. (a) Name one reagent that can be reacted with dilute hydrochloric acid to produce
Sulphur (IV) oxide
(b) What would be observed if moist blue litmus paper is dropped into a gas jar of
sulphur (IV) oxide? Explain your answer with an equation
11. (a) State two properties that vulcanized rubber posses as a result of vulcanization
(b) During Frasch process molten sulphur flows out through the middle pipe but not
through the outer pipe. Give a reason
12. (a) Give two reasons why during the manufacture of sulphuric (VI) acid, sulphur (VI) Oxide,
is dissolved in concentrated Sulphuric (VI ) acid instead of dissolving in water
b) State one use of sulphuric (VI) acid
13. The diagram below may be used to react hydrogen sulphide and sulphur (IV) oxide.
Study it and answer the questions that follow:
(a) What is observed in the jars
(b) Write an equation for the reaction
(c) What is the role of sulphur (IV) oxide in the reaction
1 4. The diagram below shows the extraction of sulphur by Frasch process.
a) State the uses of pipes A, B and C.
b) Give two crystalliric allotropes of sulphur.
c) Write an equation for the combustion of sulphur.
d) Name the product formed when a mixture of sulphur and Iron is heated.
e) Give two uses of sulphur.
f) 6.0 dm3 of sulphur (IV) oxide were oxidized by oxygen to sulphur (VI) oxide.
(i) Write an equation for the reaction.
(ii) Calculate the number of moles of sulphur (IV) oxide and oxygen used at R.T.P.
(iii) Determine the volume of oxygen used.
(Molar volume of a gas at R.T.P. is 24.0 dm3)
15. The diagrams below represent two allotropes of Sulphur. Study them and answer the questions
which follow:-
(i) Name the two allotropes labelled X and Y
(ii) (I) Explain why a piece of burning magnesium continues to burn in a gas jar of Sulphur
(IV) Oxide
(II) Explain how one of the products formed in (I) above can be obtained from the mixture
16. (a) (i) Name the two crystalline forms of sulphur
(ii) Briefly explain how plastic sulphur is formed
(b) The scheme below represents the steps followed in the contact process. Study it and answer
the questions that follow:-
(a) Name two possible identities of solid A
(b) Name one impurities removed by the purifier
(c) Why is it necessary to remove impurities?
(d) Write down the equation of the reaction taking place in the converter
(e) (I) Name the two catalysts that can be used in the converter
(II) What is the function of heat exchanger?
(f) Sulphuric (VI) Oxide is not dissolved directly into water? Explain
(g) (I) Name the main pollutant in the contact process.
(II) How can the pollution in (g) (I) above be controlled?
(h) Give one use of sulphuric (VI) acid
7. The set-up below was used to prepare dry sample of hydrogen sulphide gas
(a) (i) Complete the diagram to show how the gas was collected
(ii) Identify the following:-
I. Solid H ………………………………………………………………………………
II. Solid I ……………………………………………………………………………..
(iii) Write an equation for the reaction that occurred in the flask between solid H and dilute Hydrochloric acid
(b) When hydrogen sulphide gas was passed through a solution of Iron (III) chloride, the following
observations were made:-
(i) the colour of the solution changed from reddish-brown to green and
(ii) a yellow solid was deposited
Explain the observation
(c) In the manufacture of Sulphuric (VI) acid by contact process sulphur (IV) oxide is made to
react with air to form sulphur (VI) oxide as shown:-
(i) Name the catalyst in this reaction
(ii) State and explain the effect of the following changes on the yield of sulphur (VI) oxide
I. Increasing the pressure
II. Using a catalyst
(iii) Explain why sulphur (VI) oxide gas is absorbed in concentrated sulphur (VI) acid before
dilution
18. The flow chart below shows a sequence of chemical reactions starting with sulphur.
Study it and answer the questions that follow:-
(a) (i) State one observation made when the reaction in step 1 was in progress
(ii) Explain why dilute hydrochloric acid cannot be used in step 1
(iii) Write the equation for the reaction that took place in step 1
(iv) Name the reactions that took place in step 4
(v) Name solution A ……………………………………………………………….
(vi) State and explain the harmful effects on the environment of the gas C produced in step 1
19 a) Sulphur occurs naturally in two different forms called allotropes;
i) What are allotropes
ii) the two allotropes of sulphur are stable at different temperatures, as shown in the
equations below.
Give the name to the temperature 95.5ºC
b) below is a flow diagram for the contact process for manufacture of sulphuric acid(VI)
i) Give the name of the chambers labelled
(1½mks)
ii) State the three conditions in the converter
(1½mks)
iii) Explain why the gases are passed though:
I. The dust precipitator and drying power
II. The chamber labeled Y
(iv) Write the balanced equations for the reactions in :
Step 2
Step 3
Step 4
20. Study the figure below:
State and explain the observations made in:
Test tube L ………………………………………………………………..
Test tube K ………………………………………………………………………..
21. The set-up below was used to prepare and collect hydrogen sulphide gas. Study it and answer
the questions that follow:-
(a) Name solid V
(b) Give a reason why warm water is used in the set-up
22. Sulphur (IV) oxide and nitrogen (II) oxide are some of the gases released from internal
combustion engines. State how these gases affect the environment.
23. When hydrogen sulphide gas was bubbled into an aqueous solution of Iron (III) chloride, a
yellow precipitate was formed.
a) State another observation that was made.
b) Write an equation for the reaction that took place.
c) What type of reaction was undergone by hydrogen sulphide in this reaction?
24. In an attempt to prepare Sulphur (IV) Oxide gas, dilute Sulphuric acid was reacted
with barium carbonate. The yield of Sulphur dioxide was found to be negligible.
Explain
Subscribe to:
Post Comments (Atom)








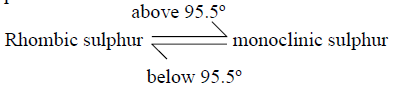












B.ed application form 2020
ReplyDeleteB.ed course duration Delhi
B.ed Admission Helpline Contact Number Jammu
CRSU B.ed college list
Kurukshetra B.ed application
this questions re very useful to people and they are of good help .Thank you so much Omugusu
ReplyDelete