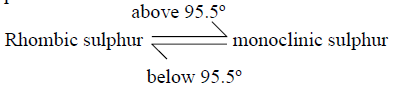1/ The BBI Report is a terrible anti-climax, and carries mixed results. This thread will interrogate those recommendations. Broadly, the recommendations do not reflect the urgency, influence and prominence that supported its mandate.
2/ The proposal to create the position Prime Minister is a huge PR exercise intended to alter the optics without meaningful drive towards political inclusion. The proposed PM is nothing more than a super CS drawn from the same government. This proposal must be rejected.
3/ The proposal that the runners up in a presidential election be the Leader of Opposition reintroduces the problem of personalization of power. Must be rejected. The present approach of second best performing party supplies the Leader of Minority in Parliament is more inclusive.
4/ The recommendation that gender representation be achieved through party lists is not new thinking. There's a court order to that effect. But the law still anticipates gender top up where the ballot does not return such number of women to meet the 2/3 gender threshold.
5/ The idea that the private sector should recruit for public service ostensibly to promote inclusivity (equal chance regardless of ethnic extraction) is untenable in the law. The National Employment Authority is best placed to oversight public service recruitment and placement.
6/ The recommendation to develop a 50-year plan to achieve shared prosperity is a curious and abrupt adjustment to Vision 2030. Shared prosperity, at the minimum, has to do with equitable sharing of available resources, and prudent and parsimonious use of those resources.
7/ The proposal to reward whistleblowers with 5% of the loot is a curious recommendation, and is inconsistent with civic virtues. Incentives for whistleblowing will have the reverse effect of undermining national ethos, and values.
8/ The recommendation that corruption be fought through public wealth declarations and resignations is a welcome move. A curious omission is the recognition that corruption is best fought at the ballot.
9/ The proposal to increase county revenue allocations from the present 15% to 35% minimum is a welcome boost for devolution. If there's political consensus, this will be BBI's biggest win. Devolution carries better potential for promoting financial and political inclusion.
10/ The proposal to disband IEBC is abrupt, illegal and unconstitutional, and must be rejected. Firing commissioners who enjoy protected tenure by altering the law to circumvent constitutional procedures for removal amounts to political interference with the independence of IEBC.
11/ The proposal to have politicians influence appointment of IEBC commissioners is illegal and unconstitutional, and must be rejected. The proposed mixed model approach will alter the approach in the constitution. The constitution supports the neutral expert approach.
12/ The constitution requires a settled approach of political neutrality at the IEBC, and all constitutional commissions. This means insulation from politics, as opposed to inoculation/injecting politics at the IEBC.
13/ If we alter the neutral expert approach in favour of the mixed model approach/IPPG format, there's the risk of interfering with the architectural integrity of the constitution (distorting the balance and stability in the constitution).
14/ The second problem with injecting politics into IEBC is the lack of a settled culture of political morality. ODM supported nominees to IEBC could change loyalty midway an election and deepen the crisis at the IEBC and plunge the country into chaos.
15/ The proposal to teach good manners and learn about different cultures in the formal education system is an excellent investment for the future.
---
In the end, BBI's biggest win is strengthening devolution. It has neglected the Judiciary, and promised to weaken IEBC.
This analysis has been done by Steve Ogolla who is a lawyer. As the editor of this blog I find a lot of sense in what Steve is saying.
Wednesday, 27 November 2019
Monday, 25 November 2019
Sulphur and its compounds
1. Sulphur is extracted from underground deposits by a process in which three concentric pipes are
sunk down to the deposits as shown below
(a) Name the process represented above
(b) What is passed down through pipe J?
(c) Name the two allotropes of sulphur
2. Commercial sulphuric acid has a density of 1.8gcm3.
(a) Calculate the molarity of this acid
(b) Determine the volume of commercial acid in (a) above that can be used to prepare
500cm3 of 0.2M H2SO4 solution
3. Oleum (H2S2O7) is an intermediate product in the industrial manufacture of sulphuric acid
(a) How is oleum converted into sulphuric (IV) acid?
(b) Give one use of sulphuric acid
4. Differentiate between the bleaching action of chloride and sulphur (IV) oxide gas.
5. (i) Is concentrated sulphuric acid a weak acid or a strong acid?
(ii) Explain your answer in (i) above.
6. In the manufacture of sulphuric acid, sulphur (IV) oxide is oxidized to sulphur (VI) oxide.
a) Name the catalyst used
b) Write the equation representing the conversion of sulphur (IV) oxide to sulphur(VI)oxide
c) Explain using equations how dilute sulphuric acid is finally obtained from sulphur (VI) oxide
7. When a mixture of concentrated sulphuric acid and copper turnings is strongly heated,
a colourless gas and solid mixture of white and black solids are formed. When this solid
mixture is treated with distilled water, and filtered, a blue solution and black solid residue
are collected. Explain the observations on the solid mixture formed in the above experiment
8. The set-up below is used to prepare dry sulphur (IV) Oxide in the laboratory. Answer questions
that follow:
(a) Identify the mistake in the set-up
(b) Write an equation for the reaction in the set-up
(c) State how the polluting effects of the gas on the environment can be controlled
9. (a) State the observation made at the end of the experiment when a mixture of iron
powder and sulphur are heated in a test-tube
(b) Write an equation for the reaction between the product in (a) above and dilute
hydrochloric acid
(c) When a mixture of iron powder and sulphur is heated it glows more brightly than
that of iron fillings and sulphur. Explain this observation
10. (a) Name one reagent that can be reacted with dilute hydrochloric acid to produce
Sulphur (IV) oxide
(b) What would be observed if moist blue litmus paper is dropped into a gas jar of
sulphur (IV) oxide? Explain your answer with an equation
11. (a) State two properties that vulcanized rubber posses as a result of vulcanization
(b) During Frasch process molten sulphur flows out through the middle pipe but not
through the outer pipe. Give a reason
12. (a) Give two reasons why during the manufacture of sulphuric (VI) acid, sulphur (VI) Oxide,
is dissolved in concentrated Sulphuric (VI ) acid instead of dissolving in water
b) State one use of sulphuric (VI) acid
13. The diagram below may be used to react hydrogen sulphide and sulphur (IV) oxide.
Study it and answer the questions that follow:
(a) What is observed in the jars
(b) Write an equation for the reaction
(c) What is the role of sulphur (IV) oxide in the reaction
1 4. The diagram below shows the extraction of sulphur by Frasch process.
a) State the uses of pipes A, B and C.
b) Give two crystalliric allotropes of sulphur.
c) Write an equation for the combustion of sulphur.
d) Name the product formed when a mixture of sulphur and Iron is heated.
e) Give two uses of sulphur.
f) 6.0 dm3 of sulphur (IV) oxide were oxidized by oxygen to sulphur (VI) oxide.
(i) Write an equation for the reaction.
(ii) Calculate the number of moles of sulphur (IV) oxide and oxygen used at R.T.P.
(iii) Determine the volume of oxygen used.
(Molar volume of a gas at R.T.P. is 24.0 dm3)
15. The diagrams below represent two allotropes of Sulphur. Study them and answer the questions
which follow:-
(i) Name the two allotropes labelled X and Y
(ii) (I) Explain why a piece of burning magnesium continues to burn in a gas jar of Sulphur
(IV) Oxide
(II) Explain how one of the products formed in (I) above can be obtained from the mixture
16. (a) (i) Name the two crystalline forms of sulphur
(ii) Briefly explain how plastic sulphur is formed
(b) The scheme below represents the steps followed in the contact process. Study it and answer
the questions that follow:-
(a) Name two possible identities of solid A
(b) Name one impurities removed by the purifier
(c) Why is it necessary to remove impurities?
(d) Write down the equation of the reaction taking place in the converter
(e) (I) Name the two catalysts that can be used in the converter
(II) What is the function of heat exchanger?
(f) Sulphuric (VI) Oxide is not dissolved directly into water? Explain
(g) (I) Name the main pollutant in the contact process.
(II) How can the pollution in (g) (I) above be controlled?
(h) Give one use of sulphuric (VI) acid
7. The set-up below was used to prepare dry sample of hydrogen sulphide gas
(a) (i) Complete the diagram to show how the gas was collected
(ii) Identify the following:-
I. Solid H ………………………………………………………………………………
II. Solid I ……………………………………………………………………………..
(iii) Write an equation for the reaction that occurred in the flask between solid H and dilute Hydrochloric acid
(b) When hydrogen sulphide gas was passed through a solution of Iron (III) chloride, the following
observations were made:-
(i) the colour of the solution changed from reddish-brown to green and
(ii) a yellow solid was deposited
Explain the observation
(c) In the manufacture of Sulphuric (VI) acid by contact process sulphur (IV) oxide is made to
react with air to form sulphur (VI) oxide as shown:-
(i) Name the catalyst in this reaction
(ii) State and explain the effect of the following changes on the yield of sulphur (VI) oxide
I. Increasing the pressure
II. Using a catalyst
(iii) Explain why sulphur (VI) oxide gas is absorbed in concentrated sulphur (VI) acid before
dilution
18. The flow chart below shows a sequence of chemical reactions starting with sulphur.
Study it and answer the questions that follow:-
(a) (i) State one observation made when the reaction in step 1 was in progress
(ii) Explain why dilute hydrochloric acid cannot be used in step 1
(iii) Write the equation for the reaction that took place in step 1
(iv) Name the reactions that took place in step 4
(v) Name solution A ……………………………………………………………….
(vi) State and explain the harmful effects on the environment of the gas C produced in step 1
19 a) Sulphur occurs naturally in two different forms called allotropes;
i) What are allotropes
ii) the two allotropes of sulphur are stable at different temperatures, as shown in the
equations below.
Give the name to the temperature 95.5ºC
b) below is a flow diagram for the contact process for manufacture of sulphuric acid(VI)
i) Give the name of the chambers labelled
(1½mks)
ii) State the three conditions in the converter
(1½mks)
iii) Explain why the gases are passed though:
I. The dust precipitator and drying power
II. The chamber labeled Y
(iv) Write the balanced equations for the reactions in :
Step 2
Step 3
Step 4
20. Study the figure below:
State and explain the observations made in:
Test tube L ………………………………………………………………..
Test tube K ………………………………………………………………………..
21. The set-up below was used to prepare and collect hydrogen sulphide gas. Study it and answer
the questions that follow:-
(a) Name solid V
(b) Give a reason why warm water is used in the set-up
22. Sulphur (IV) oxide and nitrogen (II) oxide are some of the gases released from internal
combustion engines. State how these gases affect the environment.
23. When hydrogen sulphide gas was bubbled into an aqueous solution of Iron (III) chloride, a
yellow precipitate was formed.
a) State another observation that was made.
b) Write an equation for the reaction that took place.
c) What type of reaction was undergone by hydrogen sulphide in this reaction?
24. In an attempt to prepare Sulphur (IV) Oxide gas, dilute Sulphuric acid was reacted
with barium carbonate. The yield of Sulphur dioxide was found to be negligible.
Explain
sunk down to the deposits as shown below
(a) Name the process represented above
(b) What is passed down through pipe J?
(c) Name the two allotropes of sulphur
2. Commercial sulphuric acid has a density of 1.8gcm3.
(a) Calculate the molarity of this acid
(b) Determine the volume of commercial acid in (a) above that can be used to prepare
500cm3 of 0.2M H2SO4 solution
3. Oleum (H2S2O7) is an intermediate product in the industrial manufacture of sulphuric acid
(a) How is oleum converted into sulphuric (IV) acid?
(b) Give one use of sulphuric acid
4. Differentiate between the bleaching action of chloride and sulphur (IV) oxide gas.
5. (i) Is concentrated sulphuric acid a weak acid or a strong acid?
(ii) Explain your answer in (i) above.
6. In the manufacture of sulphuric acid, sulphur (IV) oxide is oxidized to sulphur (VI) oxide.
a) Name the catalyst used
b) Write the equation representing the conversion of sulphur (IV) oxide to sulphur(VI)oxide
c) Explain using equations how dilute sulphuric acid is finally obtained from sulphur (VI) oxide
7. When a mixture of concentrated sulphuric acid and copper turnings is strongly heated,
a colourless gas and solid mixture of white and black solids are formed. When this solid
mixture is treated with distilled water, and filtered, a blue solution and black solid residue
are collected. Explain the observations on the solid mixture formed in the above experiment
8. The set-up below is used to prepare dry sulphur (IV) Oxide in the laboratory. Answer questions
that follow:
(a) Identify the mistake in the set-up
(b) Write an equation for the reaction in the set-up
(c) State how the polluting effects of the gas on the environment can be controlled
9. (a) State the observation made at the end of the experiment when a mixture of iron
powder and sulphur are heated in a test-tube
(b) Write an equation for the reaction between the product in (a) above and dilute
hydrochloric acid
(c) When a mixture of iron powder and sulphur is heated it glows more brightly than
that of iron fillings and sulphur. Explain this observation
10. (a) Name one reagent that can be reacted with dilute hydrochloric acid to produce
Sulphur (IV) oxide
(b) What would be observed if moist blue litmus paper is dropped into a gas jar of
sulphur (IV) oxide? Explain your answer with an equation
11. (a) State two properties that vulcanized rubber posses as a result of vulcanization
(b) During Frasch process molten sulphur flows out through the middle pipe but not
through the outer pipe. Give a reason
12. (a) Give two reasons why during the manufacture of sulphuric (VI) acid, sulphur (VI) Oxide,
is dissolved in concentrated Sulphuric (VI ) acid instead of dissolving in water
b) State one use of sulphuric (VI) acid
13. The diagram below may be used to react hydrogen sulphide and sulphur (IV) oxide.
Study it and answer the questions that follow:
(a) What is observed in the jars
(b) Write an equation for the reaction
(c) What is the role of sulphur (IV) oxide in the reaction
1 4. The diagram below shows the extraction of sulphur by Frasch process.
a) State the uses of pipes A, B and C.
b) Give two crystalliric allotropes of sulphur.
c) Write an equation for the combustion of sulphur.
d) Name the product formed when a mixture of sulphur and Iron is heated.
e) Give two uses of sulphur.
f) 6.0 dm3 of sulphur (IV) oxide were oxidized by oxygen to sulphur (VI) oxide.
(i) Write an equation for the reaction.
(ii) Calculate the number of moles of sulphur (IV) oxide and oxygen used at R.T.P.
(iii) Determine the volume of oxygen used.
(Molar volume of a gas at R.T.P. is 24.0 dm3)
15. The diagrams below represent two allotropes of Sulphur. Study them and answer the questions
which follow:-
(i) Name the two allotropes labelled X and Y
(ii) (I) Explain why a piece of burning magnesium continues to burn in a gas jar of Sulphur
(IV) Oxide
(II) Explain how one of the products formed in (I) above can be obtained from the mixture
16. (a) (i) Name the two crystalline forms of sulphur
(ii) Briefly explain how plastic sulphur is formed
(b) The scheme below represents the steps followed in the contact process. Study it and answer
the questions that follow:-
(a) Name two possible identities of solid A
(b) Name one impurities removed by the purifier
(c) Why is it necessary to remove impurities?
(d) Write down the equation of the reaction taking place in the converter
(e) (I) Name the two catalysts that can be used in the converter
(II) What is the function of heat exchanger?
(f) Sulphuric (VI) Oxide is not dissolved directly into water? Explain
(g) (I) Name the main pollutant in the contact process.
(II) How can the pollution in (g) (I) above be controlled?
(h) Give one use of sulphuric (VI) acid
7. The set-up below was used to prepare dry sample of hydrogen sulphide gas
(a) (i) Complete the diagram to show how the gas was collected
(ii) Identify the following:-
I. Solid H ………………………………………………………………………………
II. Solid I ……………………………………………………………………………..
(iii) Write an equation for the reaction that occurred in the flask between solid H and dilute Hydrochloric acid
(b) When hydrogen sulphide gas was passed through a solution of Iron (III) chloride, the following
observations were made:-
(i) the colour of the solution changed from reddish-brown to green and
(ii) a yellow solid was deposited
Explain the observation
(c) In the manufacture of Sulphuric (VI) acid by contact process sulphur (IV) oxide is made to
react with air to form sulphur (VI) oxide as shown:-
(i) Name the catalyst in this reaction
(ii) State and explain the effect of the following changes on the yield of sulphur (VI) oxide
I. Increasing the pressure
II. Using a catalyst
(iii) Explain why sulphur (VI) oxide gas is absorbed in concentrated sulphur (VI) acid before
dilution
18. The flow chart below shows a sequence of chemical reactions starting with sulphur.
Study it and answer the questions that follow:-
(a) (i) State one observation made when the reaction in step 1 was in progress
(ii) Explain why dilute hydrochloric acid cannot be used in step 1
(iii) Write the equation for the reaction that took place in step 1
(iv) Name the reactions that took place in step 4
(v) Name solution A ……………………………………………………………….
(vi) State and explain the harmful effects on the environment of the gas C produced in step 1
19 a) Sulphur occurs naturally in two different forms called allotropes;
i) What are allotropes
ii) the two allotropes of sulphur are stable at different temperatures, as shown in the
equations below.
Give the name to the temperature 95.5ºC
b) below is a flow diagram for the contact process for manufacture of sulphuric acid(VI)
i) Give the name of the chambers labelled
(1½mks)
ii) State the three conditions in the converter
(1½mks)
iii) Explain why the gases are passed though:
I. The dust precipitator and drying power
II. The chamber labeled Y
(iv) Write the balanced equations for the reactions in :
Step 2
Step 3
Step 4
20. Study the figure below:
State and explain the observations made in:
Test tube L ………………………………………………………………..
Test tube K ………………………………………………………………………..
21. The set-up below was used to prepare and collect hydrogen sulphide gas. Study it and answer
the questions that follow:-
(a) Name solid V
(b) Give a reason why warm water is used in the set-up
22. Sulphur (IV) oxide and nitrogen (II) oxide are some of the gases released from internal
combustion engines. State how these gases affect the environment.
23. When hydrogen sulphide gas was bubbled into an aqueous solution of Iron (III) chloride, a
yellow precipitate was formed.
a) State another observation that was made.
b) Write an equation for the reaction that took place.
c) What type of reaction was undergone by hydrogen sulphide in this reaction?
24. In an attempt to prepare Sulphur (IV) Oxide gas, dilute Sulphuric acid was reacted
with barium carbonate. The yield of Sulphur dioxide was found to be negligible.
Explain
Organic chemistry 1
1. Use the flow chart below to answer the questions that follow:
(a) What observation would be made in process K?
(b) Name another conditions necessary for process J to take place
(c) Give the name of substance V
2. But-z-ene undergoes hydrogenation according to the equation given below
CH3CH = CHCH3 (g) + H2(g)-----------> CH3CH2CH2CH3(g)
(a) Name the product formed when but-z-ene reacts with hydrogen gas
(b) State one industrial use of hydrogenation
3. Write the structures of the following compounds:-
(a) But—2-yne
(b) 2,2-dimethylpropane
4. a)What is meant by Isomerism?
b) Draw and name two Isomers of butane.
5. Study the information in the table below and answer the questions that follow:
b) Give the group and period to which elements Q and R respectively.
Q ……………………………………………………
R ……………………………………………………
6. Compound W reacted with chlorine to form compound X only. The structural formula of
X is shown below:
(a) Give the structural formula and name of compound W
(b) Name compound X ……………………………………………………………………
7. In petrol chemical industries, long chain alkanes are broken down in to simpler substances
in a process called cracking
a) Why is cracking necessary?
b) State the two conditions required in cracking
c) Draw the structure of 1-chloro-2, 2-dimethylpropane
8. In a reaction an alcohol K was converted to hex-1-ene
a) Name reagent and condition necessary for the reaction in 6 (a) above to occur
9. (a) Give the IUPAC systematic names of compounds Q and R
Q: CH2CHClCHlCH2CH3
R: CH3CHClCH2ClCH3
(b) The organic compounds Q and R in (b) above, are formed when one mole of hydrocarbon
N reacts with two moles of hydrogen chloride gas;
(i) Structural formula of N
(ii) The IUPAC systematic name of N
10. Distinguish between the isotopes and isomers
11. Polymerisation of ethene takes place as shown in the equation below
Name the type of polymerisation undergone by ethene in the reaction above
12. (a) State Gay Lussac’s law
13. 10cm3 of methane (CH4) gas is exploded with 150cm3 of air containing 20% oxygen
and 80% nitrogen. The products were allowed to cool to room temperature. What will
be the total volume of the gases at the end of the reaction?
14. Give the open structures of:-
(i) 3-chlorohex-l-yne
(ii) CH3OH
15. A fixed mass of gas occupies 105cm3 at -14ºC and 650mmHg pressure. At what temperature in
degrees Celsius will it have a volume of 15cm3 if the pressure is adjusted to 690mmHg pressure?
16. Write an equation for the reaction that takes place between ethene and concentrated
Sulphuric (VI) acid
17. Petroleum (crude oil) is a mixture of several compounds which are separated in a Changamwe
refinery by means of apparatus as shown below:
(a) (i) What is the name of the apparatus above
(ii) What is the name of the process which is used in separation of crude oil
(iii) What physical property of compounds in the mixture does the separation depend
(iv) Use the letter A to G to describe where the following could be formed:.
I. The fraction that represents gases
II. The fraction that represents the largest molecules
III. The fraction that represents liquids with the lowest boiling points
(b) State the use of product produce at
G………………………………………………………………………………………
C……………………………………………………………………………………….
(c) Draw apparatus for the separation of the product produce at D and water
18. Study the flow chart below and answer the questions that follow:-
(i) Give the name of the substance CH=CH …………………………………………
(ii) To which group of hydrocarbons does the substance in (i) above belong?
(iii) Give two reagents that can be used to prepare the substance named in (i) above
(iv) State two physical properties of the substances in (i) above
(v) Give the names to the process in step I and 2
(vi) Write an equation to show how substance A is formed
(iv) Identify substance B ……………………………………………………
19. The diagram below represents a large-scale fractional distillation plant used to separate
the components A, B, C and D in a mixture
(a) The components have the following average relative molecular masses not necessarily in that
order; 282, 184, 44 and 128.
(a) (i) What is the physical state of B at the position marked Q?
(ii) Which component has an average relative molecular mass of 128? Explain
(iii) State with a reason whether C is pure or impure
(iv) Explain how the mixture is separated into its components
(v) Name two naturally occurring mixtures that are separated using this process
20. The diagram below represents a large-scale fractional distillation plant used to separate
the components A, B, C and D in a mixture
(a) The components have the following average relative molecular masses not necessarily in that
order; 282, 184, 44 and 128.
(a) (i) What is the physical state of B at the position marked Q?
(ii) Which component has an average relative molecular mass of 128? Explain
(iii) State with a reason whether C is pure or impure
(iv) Explain how the mixture is separated into its components
(v) Name two naturally occurring mixtures that are separated using this process
21. a) The table below gives information about the major constituents of crude oil. Study it and
answer the questions that follow:
i) Which of the constituents of crude has molecules with the highest number of carbon
atoms? Explain
ii) Name the process you would use to separate a mixture of petrol and diesel and explain how
the separation takes place
iii) Explain why the constituents of crude oil do not have a sharp boiling point
iv) Name the gas that is likely to be a constituent of crude oil and write its formula
b) i) What condition could cause a poisonous gas to be formed when kerosene is burnt.
Explain
ii) Give one use of bitumen
22. (a) The set-up below was used to prepare ethyne gas
(i) Identify solid E
(ii) Complete the diagram to show how the gas can be collected
(iii) Write an equation to show how the gas is formed
(iv) Complete the equation below: )
(v) What is the role of sand in the experiment?
(b) (i) Explain the meaning of esterification
(ii) Complete the equation below :
(iii) What type of reaction is occurring above
(c) Given the reaction:
(i) Identify substance:
F………………………………… N………………………………
(ii) Name the process represented above?
(d) Give one use of substance N
(i) Name another source of hydrogen apart from electrolysis of water
(ii) What conditions are necessary for step III to occur?
(iii) Write the equation for the formation of colourless gas Q
(iv) Give one use of nitric (V) acid
(b) State and explain the observations that would be made if a sample of copper metal is
heated with concentrated nitric (V) acid
24. (a) Give the systematic names of the following compounds:-
(b) State the observations made when buton-l-ol reacts with:-
(i) Acidified potassium dichromate (VI) solution
(ii) Potassium metal
(c) Ethanol obtained from glucose can be converted to ethene as shown below:-
Name and describe the processes that take place in steps I and II
(d) Compounds A and B have the same molecular formula C3H6O2. Compound A librates
Carbon (IV) Oxide on addition of aqueous sodium carbonate while compound B does not.
Compound B has a sweet smell. Draw the possible structures of:-
(e) Give two ways how the disposal of polymers such as polychloroethene by burning pollutes
the environment
25. (a) Name the following compounds (CH3)3 C CH2 CH2 CH3
Use the flow chart below to answer the questions that follow:-
(b) (i) Name the following :-
I. Gas S ……………………………………………………………….……. ( )
II. Gas P …………………………………………………………………
III. J ……………………………………………………………………….
(ii) Name the processes involved in the following steps:
I. Step I …………………………………………………………………………..
II. Step II …………………………………………………………………………….
III. Step III …………………………………………………………………………….
(iii) Write a chemical equation for the complete combustion of substance M
(iv) Name the condition and reagent in step III
Condition ……………………………………………………………………………………
Reagent …………………………………………………………………………………….
(v) Calculate the mass of salt R that would be formed by using 21.9 tonnes of N when it reacts
with excess sodium hydroxide ( C= 12.0 H= 1.0 Na = 23)
(vi) Draw the structure of polymer K
II. State one use of the above polymer
………………………………………………………………………………………………….
(c) (i) Name the class to which the following cleansing agents belong:-
II. Which cleaning agent above is not environmental friendly? Explain
26. The molecular formula of a hydrocarbon is C6H14. The hydrocarbon can be converted into two
other hydrocarbon as shown by the equation below:
(i) Name and draw the possible structural formula of X
(ii) State and explain the observations that would be made if a few drops of bromine water
were added to a sample of X
(iii) Write an equation for the complete combustion of C3H8
27. (a) Give the names of the following
(i) CH3CH2CH3
(ii) CH3CCCH3
(b) Ethene is used in making polyethene bag in a process called polymerization
(i) Name the type of polymer that is formed when ethane polymerise
(ii) Describe a simple chemical test that can be used to identify ethane gas in the laboratory
(c) Study the information in the table below and answer the questions that follow:-
i. Write the general formula of the hydrocarbons in the table above
ii. Determine the molecular of a hydrocarbon with 5 carbon atoms and draw its structural formula
Molecular formula
Structural formula
(d) Study the scheme below and answer the questions that follow
(i) Name the reagents in
Step I ……………………………
Step II ……………………………
Step IV ………………
(ii) Write an equation for the complete combustion of CH CH
(iii) Give two uses of CH4
28. Give the systematic names of the following compounds;
29. Study the data given in the following table and answer the questions that follow. The letters
are not the actual symbols of elements.
(i) State and explain the trend in melting point in A B C
(ii) Explain why the melting point and boiling points of element D is the highest
(iii) Explain why the element represented by letter E has two melting point values
(iv) Write down the chemical formula between element C and sulphate ions
(v) Name the chemical family in which H belong and state one use of the element
(vi) What is the nature of the oxide of the elements represented by letters C and F?
30. a) The table below gives information about the major constituents of crude oil. Study it and
answer the questions that follow:
i) Which of the constituents of crude has molecules with the highest number of carbon
atoms? Explain
ii) Name the process you would use to separate a mixture of petrol and diesel and explain how
the separation takes place
iii) Explain why the constituents of crude oil do not have a sharp boiling point
iv) Name the gas that is likely to be a constituent of crude oil and write its formula
b) i) What condition could cause a poisonous gas to be formed when kerosene is burnt.
Explain
ii) Give one use of bitumen
31. Study the information in the table below and answer the questions that follow
i) Write the general formula of the hydrocarbons in the table
ii) Predict the relative atomic mass of the hydrocarbons with 5 carbon atoms
iii) Determine the relative atomic mass of the hydrocarbon in (ii) above and draw its
structural formula (H=1.0, C=12.0)
32. Substance “M” with a general formula C2Hy burnt in chlorine gas with a red flame producing
a cloud of black specks and colourless gas G.
(a) State the collective name for compounds which ‘M’ belongs
(b) With reason, state the identity of the black specks and colour gas “G”.
33. 2.63g of a solution of sodium chloride at 20.0oC was reacted with silver nitrate. After filtration,
washing and drying, 2.36g of silver chloride was obtained. Determine the solubility of sodium
chloride at 20.0oC . (Na=23, Cl= 35.5, Ag = 108)
(b) Determine the number of moles of carbon (IV) Oxide gas produced when sodium
carbonate reacted with dilute sulphuric (VI) acid (Molar gas volume =24dm3)
34. Write down all the isomers of but-z-ene and give their IUPAC names
35. (a) A hydrocarbon compound Z decolourizes bromine liquid in the presence of light but
does not decolourize acidified potassium manganate (VII). Name and draw the structural
formula of the eighth member of this homologous series
36. (a) What is meant by isomerism?
(b) Draw and name two isomers of Butyne
(a) What observation would be made in process K?
(b) Name another conditions necessary for process J to take place
(c) Give the name of substance V
2. But-z-ene undergoes hydrogenation according to the equation given below
CH3CH = CHCH3 (g) + H2(g)-----------> CH3CH2CH2CH3(g)
(a) Name the product formed when but-z-ene reacts with hydrogen gas
(b) State one industrial use of hydrogenation
3. Write the structures of the following compounds:-
(a) But—2-yne
(b) 2,2-dimethylpropane
4. a)What is meant by Isomerism?
b) Draw and name two Isomers of butane.
5. Study the information in the table below and answer the questions that follow:
b) Give the group and period to which elements Q and R respectively.
Q ……………………………………………………
R ……………………………………………………
6. Compound W reacted with chlorine to form compound X only. The structural formula of
X is shown below:
(a) Give the structural formula and name of compound W
(b) Name compound X ……………………………………………………………………
7. In petrol chemical industries, long chain alkanes are broken down in to simpler substances
in a process called cracking
a) Why is cracking necessary?
b) State the two conditions required in cracking
c) Draw the structure of 1-chloro-2, 2-dimethylpropane
8. In a reaction an alcohol K was converted to hex-1-ene
a) Name reagent and condition necessary for the reaction in 6 (a) above to occur
9. (a) Give the IUPAC systematic names of compounds Q and R
Q: CH2CHClCHlCH2CH3
R: CH3CHClCH2ClCH3
(b) The organic compounds Q and R in (b) above, are formed when one mole of hydrocarbon
N reacts with two moles of hydrogen chloride gas;
(i) Structural formula of N
(ii) The IUPAC systematic name of N
10. Distinguish between the isotopes and isomers
11. Polymerisation of ethene takes place as shown in the equation below
Name the type of polymerisation undergone by ethene in the reaction above
12. (a) State Gay Lussac’s law
13. 10cm3 of methane (CH4) gas is exploded with 150cm3 of air containing 20% oxygen
and 80% nitrogen. The products were allowed to cool to room temperature. What will
be the total volume of the gases at the end of the reaction?
14. Give the open structures of:-
(i) 3-chlorohex-l-yne
(ii) CH3OH
15. A fixed mass of gas occupies 105cm3 at -14ºC and 650mmHg pressure. At what temperature in
degrees Celsius will it have a volume of 15cm3 if the pressure is adjusted to 690mmHg pressure?
16. Write an equation for the reaction that takes place between ethene and concentrated
Sulphuric (VI) acid
17. Petroleum (crude oil) is a mixture of several compounds which are separated in a Changamwe
refinery by means of apparatus as shown below:
(a) (i) What is the name of the apparatus above
(ii) What is the name of the process which is used in separation of crude oil
(iii) What physical property of compounds in the mixture does the separation depend
(iv) Use the letter A to G to describe where the following could be formed:.
I. The fraction that represents gases
II. The fraction that represents the largest molecules
III. The fraction that represents liquids with the lowest boiling points
(b) State the use of product produce at
G………………………………………………………………………………………
C……………………………………………………………………………………….
(c) Draw apparatus for the separation of the product produce at D and water
18. Study the flow chart below and answer the questions that follow:-
(i) Give the name of the substance CH=CH …………………………………………
(ii) To which group of hydrocarbons does the substance in (i) above belong?
(iii) Give two reagents that can be used to prepare the substance named in (i) above
(iv) State two physical properties of the substances in (i) above
(v) Give the names to the process in step I and 2
(vi) Write an equation to show how substance A is formed
(iv) Identify substance B ……………………………………………………
19. The diagram below represents a large-scale fractional distillation plant used to separate
the components A, B, C and D in a mixture
(a) The components have the following average relative molecular masses not necessarily in that
order; 282, 184, 44 and 128.
(a) (i) What is the physical state of B at the position marked Q?
(ii) Which component has an average relative molecular mass of 128? Explain
(iii) State with a reason whether C is pure or impure
(iv) Explain how the mixture is separated into its components
(v) Name two naturally occurring mixtures that are separated using this process
20. The diagram below represents a large-scale fractional distillation plant used to separate
the components A, B, C and D in a mixture
(a) The components have the following average relative molecular masses not necessarily in that
order; 282, 184, 44 and 128.
(a) (i) What is the physical state of B at the position marked Q?
(ii) Which component has an average relative molecular mass of 128? Explain
(iii) State with a reason whether C is pure or impure
(iv) Explain how the mixture is separated into its components
(v) Name two naturally occurring mixtures that are separated using this process
21. a) The table below gives information about the major constituents of crude oil. Study it and
answer the questions that follow:
i) Which of the constituents of crude has molecules with the highest number of carbon
atoms? Explain
ii) Name the process you would use to separate a mixture of petrol and diesel and explain how
the separation takes place
iii) Explain why the constituents of crude oil do not have a sharp boiling point
iv) Name the gas that is likely to be a constituent of crude oil and write its formula
b) i) What condition could cause a poisonous gas to be formed when kerosene is burnt.
Explain
ii) Give one use of bitumen
22. (a) The set-up below was used to prepare ethyne gas
(i) Identify solid E
(ii) Complete the diagram to show how the gas can be collected
(iii) Write an equation to show how the gas is formed
(iv) Complete the equation below: )
(v) What is the role of sand in the experiment?
(b) (i) Explain the meaning of esterification
(ii) Complete the equation below :
(iii) What type of reaction is occurring above
(c) Given the reaction:
(i) Identify substance:
F………………………………… N………………………………
(ii) Name the process represented above?
(d) Give one use of substance N
(i) Name another source of hydrogen apart from electrolysis of water
(ii) What conditions are necessary for step III to occur?
(iii) Write the equation for the formation of colourless gas Q
(iv) Give one use of nitric (V) acid
(b) State and explain the observations that would be made if a sample of copper metal is
heated with concentrated nitric (V) acid
24. (a) Give the systematic names of the following compounds:-
(b) State the observations made when buton-l-ol reacts with:-
(i) Acidified potassium dichromate (VI) solution
(ii) Potassium metal
(c) Ethanol obtained from glucose can be converted to ethene as shown below:-
Name and describe the processes that take place in steps I and II
(d) Compounds A and B have the same molecular formula C3H6O2. Compound A librates
Carbon (IV) Oxide on addition of aqueous sodium carbonate while compound B does not.
Compound B has a sweet smell. Draw the possible structures of:-
(e) Give two ways how the disposal of polymers such as polychloroethene by burning pollutes
the environment
25. (a) Name the following compounds (CH3)3 C CH2 CH2 CH3
Use the flow chart below to answer the questions that follow:-
(b) (i) Name the following :-
I. Gas S ……………………………………………………………….……. ( )
II. Gas P …………………………………………………………………
III. J ……………………………………………………………………….
(ii) Name the processes involved in the following steps:
I. Step I …………………………………………………………………………..
II. Step II …………………………………………………………………………….
III. Step III …………………………………………………………………………….
(iii) Write a chemical equation for the complete combustion of substance M
(iv) Name the condition and reagent in step III
Condition ……………………………………………………………………………………
Reagent …………………………………………………………………………………….
(v) Calculate the mass of salt R that would be formed by using 21.9 tonnes of N when it reacts
with excess sodium hydroxide ( C= 12.0 H= 1.0 Na = 23)
(vi) Draw the structure of polymer K
II. State one use of the above polymer
………………………………………………………………………………………………….
(c) (i) Name the class to which the following cleansing agents belong:-
II. Which cleaning agent above is not environmental friendly? Explain
26. The molecular formula of a hydrocarbon is C6H14. The hydrocarbon can be converted into two
other hydrocarbon as shown by the equation below:
(i) Name and draw the possible structural formula of X
(ii) State and explain the observations that would be made if a few drops of bromine water
were added to a sample of X
(iii) Write an equation for the complete combustion of C3H8
27. (a) Give the names of the following
(i) CH3CH2CH3
(ii) CH3CCCH3
(b) Ethene is used in making polyethene bag in a process called polymerization
(i) Name the type of polymer that is formed when ethane polymerise
(ii) Describe a simple chemical test that can be used to identify ethane gas in the laboratory
(c) Study the information in the table below and answer the questions that follow:-
i. Write the general formula of the hydrocarbons in the table above
ii. Determine the molecular of a hydrocarbon with 5 carbon atoms and draw its structural formula
Molecular formula
Structural formula
(d) Study the scheme below and answer the questions that follow
(i) Name the reagents in
Step I ……………………………
Step II ……………………………
Step IV ………………
(ii) Write an equation for the complete combustion of CH CH
(iii) Give two uses of CH4
28. Give the systematic names of the following compounds;
29. Study the data given in the following table and answer the questions that follow. The letters
are not the actual symbols of elements.
|
Element
|
Number of protons
|
Melting point
|
Bpt oC
|
|
A
|
11
|
98
|
890
|
|
B
|
12
|
650
|
1110
|
|
C
|
13
|
60
|
2470
|
|
D
|
14
|
1410
|
2360
|
|
E
|
15
|
442
590
|
280
|
|
F
|
16
|
113
119
|
119
|
|
G
|
17
|
-101
|
-35
|
|
H
|
18
|
-189
|
-186
|
(i) State and explain the trend in melting point in A B C
(ii) Explain why the melting point and boiling points of element D is the highest
(iii) Explain why the element represented by letter E has two melting point values
(iv) Write down the chemical formula between element C and sulphate ions
(v) Name the chemical family in which H belong and state one use of the element
(vi) What is the nature of the oxide of the elements represented by letters C and F?
30. a) The table below gives information about the major constituents of crude oil. Study it and
answer the questions that follow:
i) Which of the constituents of crude has molecules with the highest number of carbon
atoms? Explain
ii) Name the process you would use to separate a mixture of petrol and diesel and explain how
the separation takes place
iii) Explain why the constituents of crude oil do not have a sharp boiling point
iv) Name the gas that is likely to be a constituent of crude oil and write its formula
b) i) What condition could cause a poisonous gas to be formed when kerosene is burnt.
Explain
ii) Give one use of bitumen
31. Study the information in the table below and answer the questions that follow
i) Write the general formula of the hydrocarbons in the table
ii) Predict the relative atomic mass of the hydrocarbons with 5 carbon atoms
iii) Determine the relative atomic mass of the hydrocarbon in (ii) above and draw its
structural formula (H=1.0, C=12.0)
32. Substance “M” with a general formula C2Hy burnt in chlorine gas with a red flame producing
a cloud of black specks and colourless gas G.
(a) State the collective name for compounds which ‘M’ belongs
(b) With reason, state the identity of the black specks and colour gas “G”.
33. 2.63g of a solution of sodium chloride at 20.0oC was reacted with silver nitrate. After filtration,
washing and drying, 2.36g of silver chloride was obtained. Determine the solubility of sodium
chloride at 20.0oC . (Na=23, Cl= 35.5, Ag = 108)
(b) Determine the number of moles of carbon (IV) Oxide gas produced when sodium
carbonate reacted with dilute sulphuric (VI) acid (Molar gas volume =24dm3)
34. Write down all the isomers of but-z-ene and give their IUPAC names
35. (a) A hydrocarbon compound Z decolourizes bromine liquid in the presence of light but
does not decolourize acidified potassium manganate (VII). Name and draw the structural
formula of the eighth member of this homologous series
36. (a) What is meant by isomerism?
(b) Draw and name two isomers of Butyne
The mole
1. In an experiment magnesium ribbon was heated in air. The product formed was found to be
heavier than the original ribbon. Potassium manganate (VII) was on the other hand, heated in
air and product formed was found to be lighter. Explain the differences on the observation made
2. In a filtration experiment 25cm3 of a solution of Sodium Hydroxide containing 8g per
litre was required for complete neutralization of 0.245g of a dibasic acid. Calculate
the relative molecular mass of the acid (Na = 23.0, O = 16, H= 1)
3. D grams of Potassium hydroxide were dissolved is distilled water to make 100cm3 of solution.
50cm3 of the solution required 50cm3 of 2.0M nitric acid for complete neutralization.
Calculate the mass D of Potassium hydroxide (RFM of KOH = 56)
KOH(aq) + HNO3(aq)------------> KNO3(aq) + H2O(l)
4. When excess dilute hydrochloric acid was added to sodium sulphite, 960cm3 of sulphuric
(IV) Oxide gas was produced. Calculate the mass of sodium sulphate that was used.
(Molar gas volume = 24000cm3 and Molar mass of sulphite = 126g)
5. The equation of the formation of iron (III) chloride is
2Fe(s) + 3Cl2(g)--------------> 2FeCl3
Calculate the volume of chlorine which will react with iron to form 0.5g of Iron (III) chloride.
(Fe = 56 Cl=35.5). Molar gas volume at 298K = 24dm3)
6. 15.0cm3 of ethanoic acid (CH3COOH) was dissolved in water to make 500cm3 of solution.
Calculate the concentration of the solution in moles per litre
[C=12, H = 1, O = 16, density of ethanoic acid is 1.05g/cm3]
7. When 1.675g of hydrated sodium carbonate was reacted with excess hydrochloric acid,
the volume carbon (IV) oxide gas obtained at room temperature and pressure was 150cm3.
Calculate the number of moles of water of crystallization in one mole of hydrated sodium
carbonate:- (Na=23, H =1, C=12, O=16, MGV at R.T.P = 24000cm3)
8. How many chloride ions are present in 1.7g of magnesium chloride crystals?
(Avogadro’s constant = 6.0 x 1023, Mg = 24, Cl = 35.5)
9. 0.84g of aluminium reacted completely with chlorine gas. Calculate the volume of chlorine
gas used (Molar gas volume is 24dm3, Al = 27)
10. 6.4g of a mixture of sodium carbonate and sodium chloride was dissolved in water to make
50cm3 solution. 25cm3 of the solution was neutralized by 40cm3 of 0.1M HCl(aq). What is
he percentage of sodium chloride in the solid mixture?
11 An unknown mass, x, of anhydrous potassium carbonate was dissolved in water and the
solution made up to 200cm3. 25cm3 of this solution required 18cm3 of 0.22M nitric (V) acid for
complete neutralization. Determine the value of x. (K=39.0, C =12.0, O =16.0)
12. Calculate the volume of oxygen gas used during the burning of magnesium (O = 16, molar
gas volume = 24,000cm3 at room temperature)
13. A hydrated salt has the following composition by mass. Iron 20.2 %, oxygen 23.0%,
sulphur 11.5%, water 45.3%
i) Determine the formula of the hydrated salt (Fe=56, S=32, O=16, H=11)
ii) 6.95g of the hydrated salt in c(i) above were dissolved in distilled water and the total
volume made to 250cm3 of solution. Calculate the concentration of the resulting salt solution
in moles per litre. (Given that the molecula mass of the salt is 278)
14. (i) Lead (II) ions react with iodide ions according to the equation;
300cm3 of a 0.1m solution of iodide ions was added to a solution containing excess lead II ions.
Calculate the mass in grams of lead II iodide formed
(ii) Identify the colour of the product formed in (d) (i)
15. a) The diagram below represents part of the structure of sodium chloride crystal
The position of one of the sodium ions in the crystal is shown as;
i) On the diagram, mark the positions of the other three sodium ions
ii) The melting and boiling points of sodium chloride are 801C and 1413C respectively. Explain
why sodium chloride does not conduct electricity at 25C, but does not at temperatures
between 801C and 1413C
b) Give a reason why ammonia gas is highly soluble in water
c) The structure of ammonium ion is shown below;
Name the type of bond represented in the diagram by N H
d) Carbon exists in different crystalline forms. Some of these forms were recently discovered
in soot and are called fullerenes
i) What name is given to different crystalline forms of the same element
ii) Fullerenes dissolve in methylbenzene while the other forms of carbon do not. Given that soot is
a mixture of fullerenes and other solid forms of carbon, describe how crystals of fullerenes can
be obtained from soot
iii) The relative molecular mass of one of the fullerenes is 720. What is the molecular mass of
this fullerene
16. Calculate the volume of oxygen gas used during the burning of magnesium (O = 16, molar
gas volume = 24,000cm3 at room temperature)
17. Study the information in the table below and answer the questions that follow
i) Write the general formula of the hydrocarbons in the table
ii) Predict the relative atomic mass of the hydrocarbons with 5 carbon atoms
iii) Determine the relative atomic mass of the hydrocarbon in (ii) above and draw its
structural formula (H=1.0, C=12.0)
18. A hydrated salt has the following composition by mass. Iron 20.2 %, oxygen 23.0%,
sulphur 11.5%, water 45.3%
i) Determine the formula of the hydrated salt (Fe=56, S=32, O=16, H=11) (3 mks)
ii) 6.95g of the hydrated salt in c(i) above were dissolved in distilled water and the total
volume made to 250cm3 of solution. Calculate the concentration of the resulting salt solution
in moles per litre. (Given that the molecula mass of the salt is 278)
19. a) Galvanized iron sheets are made by dipping the sheets in molten Zinc.
i) Explain how zinc protects iron from rusting
ii) Name the process applied in galvanization of iron with zinc
20. Calculate the percentage of copper in 1.0g of the alloy
(Cu = 63.5 Mg = 24)
21. A factory uses nitric acid and ammonia gas as the only reactant for the preparation of the
fertilizer if the daily production of the fertilizer is 4800kg. Calculate the mass of ammonia
gas used daily
(N = 14.0, O= 16.0, H = 1.0)
22. Calculate the volume of sulphur (VI) oxide gas that would be required to produce 178kg of
oleum in step 3 molar gas volume at s.t.p = 22.4 litres H = 1 O = 16 S = 32
23. Using the answer in d (ii) above, determine:
i) The volume of 1M nitric acid that would react completely with one mole of copper
(Cu = 63.5)
ii) The volume of Nitrogen (IV) oxide gas produced when one mole of copper reacts
with excess 1M nitric acid at room temperature
24. A sample of biogas contains 35.2% by mass of methane. A biogas cylinder contains 5.0kg
of the gas. Calculate:
(i) Number of moles of methane in the cylinder (Molar mass of methane = 16)
(ii) Total volume of carbon (IV) oxide produced by the combustion of methane in the cylinder
(Molar gas volume = 24.0dm3 at room temperature and pressure)
25. 0.84g of aluminium were reacted completely with chlorine gas. Calculate the volume
of chlorine gas used. (Molar gas volume is 24dm3, Al = 27)
26. 3.52g of Carbon (IV) Oxide and 1.40g of water are produced when a mass of a hydrocarbon
is completely burnt in oxygen. Determine the empirical formula of the hydrocarbon;
(H = 1 , C= 12, O = 16)
27. Calculate the number of water molecules when 34.8g Na2CO3 xH2O is heated and 15.9g of
anhydrous Na2CO3 obtained (H=1, O=16, Na= 23, C = 12)
28. A weighed sample of crystallined sodium carbonate (Na2CO3nH2O) was heated in a crucible
until there was no further change in mass. The mass of the sample reduced by 14.5%. Calculate
the number of moles (n) of water of crystallization (Na = 23, O = 16, C = 12, H = 1)
29. In a reaction 20cm3 of 0.1 M Sodium Carbonate completely reacted with 13cm3 of dilute sulphuric acid. Find the molarity of the sulphuric acid used.
30. An organic compound P contains 68.9% carbon, 13.5% hydrogen and 21.6% oxygen.
The relative formula mass of p is 74. Determine its molecular formula. [C=12, H=1, 0=16]
31. Campers GAZ cylinder contains about 1.12dm3 of butane measured at 0o and 1atm. Given that
25% of heat is lost, what is the maximum volume of water at room temperature which can be
boiled to 100oC in order to make some coffee?
(Specific heat capacity of water = 4.2J g-1C-0c, density of water 1gcm-3 Molar gas volume 22.41 at s.t.p)
32. An aqueous solution containing anhydrous sodium carbonate was prepared by dissolving
19.6g of the salt in 250cm3 of distilled. Calculate the volume of 2M of magnesium chloride
solution required to precipitate all the carbonate ions in the solution.
(Na=23, C= 12; O = 16; Mg = 24; Cl =35.5)
33. 10.08g of ethanedioic acid (H2C2O4.xH2O) crystals were dissolved in water and made to
1dm3 solution. 25.0cm3 of this solution was completely neutralized by 20cm3 of 0.2M
sodium hydroxide solution.
Calculate
i) Molarity of the acid
ii)the value of x in H2C2O4xH2O acid
34. 1.6g of magnesium metal is reacted with excess hydrochloric acid. Calculate the volume
of hydrogen gas produced
(Molar gas volume at stp = 22.4dm3 Mg=24)
35. 60 litres of sulphur(IV) oxide were made to react with 40 litres of oxygen.
a) Which reactant was in excess and by how much?
b) What is the volume of the product?
36. During welding of cracked railway lines by thermite 12.0g of oxide of iron is reduced by
aluminium to 8.40g of iron. Determine the empirical formula of the oxide
(Fe= 56.0, O= 16.0)
heavier than the original ribbon. Potassium manganate (VII) was on the other hand, heated in
air and product formed was found to be lighter. Explain the differences on the observation made
2. In a filtration experiment 25cm3 of a solution of Sodium Hydroxide containing 8g per
litre was required for complete neutralization of 0.245g of a dibasic acid. Calculate
the relative molecular mass of the acid (Na = 23.0, O = 16, H= 1)
3. D grams of Potassium hydroxide were dissolved is distilled water to make 100cm3 of solution.
50cm3 of the solution required 50cm3 of 2.0M nitric acid for complete neutralization.
Calculate the mass D of Potassium hydroxide (RFM of KOH = 56)
KOH(aq) + HNO3(aq)------------> KNO3(aq) + H2O(l)
4. When excess dilute hydrochloric acid was added to sodium sulphite, 960cm3 of sulphuric
(IV) Oxide gas was produced. Calculate the mass of sodium sulphate that was used.
(Molar gas volume = 24000cm3 and Molar mass of sulphite = 126g)
5. The equation of the formation of iron (III) chloride is
2Fe(s) + 3Cl2(g)--------------> 2FeCl3
Calculate the volume of chlorine which will react with iron to form 0.5g of Iron (III) chloride.
(Fe = 56 Cl=35.5). Molar gas volume at 298K = 24dm3)
6. 15.0cm3 of ethanoic acid (CH3COOH) was dissolved in water to make 500cm3 of solution.
Calculate the concentration of the solution in moles per litre
[C=12, H = 1, O = 16, density of ethanoic acid is 1.05g/cm3]
7. When 1.675g of hydrated sodium carbonate was reacted with excess hydrochloric acid,
the volume carbon (IV) oxide gas obtained at room temperature and pressure was 150cm3.
Calculate the number of moles of water of crystallization in one mole of hydrated sodium
carbonate:- (Na=23, H =1, C=12, O=16, MGV at R.T.P = 24000cm3)
8. How many chloride ions are present in 1.7g of magnesium chloride crystals?
(Avogadro’s constant = 6.0 x 1023, Mg = 24, Cl = 35.5)
9. 0.84g of aluminium reacted completely with chlorine gas. Calculate the volume of chlorine
gas used (Molar gas volume is 24dm3, Al = 27)
10. 6.4g of a mixture of sodium carbonate and sodium chloride was dissolved in water to make
50cm3 solution. 25cm3 of the solution was neutralized by 40cm3 of 0.1M HCl(aq). What is
he percentage of sodium chloride in the solid mixture?
11 An unknown mass, x, of anhydrous potassium carbonate was dissolved in water and the
solution made up to 200cm3. 25cm3 of this solution required 18cm3 of 0.22M nitric (V) acid for
complete neutralization. Determine the value of x. (K=39.0, C =12.0, O =16.0)
12. Calculate the volume of oxygen gas used during the burning of magnesium (O = 16, molar
gas volume = 24,000cm3 at room temperature)
13. A hydrated salt has the following composition by mass. Iron 20.2 %, oxygen 23.0%,
sulphur 11.5%, water 45.3%
i) Determine the formula of the hydrated salt (Fe=56, S=32, O=16, H=11)
ii) 6.95g of the hydrated salt in c(i) above were dissolved in distilled water and the total
volume made to 250cm3 of solution. Calculate the concentration of the resulting salt solution
in moles per litre. (Given that the molecula mass of the salt is 278)
14. (i) Lead (II) ions react with iodide ions according to the equation;
300cm3 of a 0.1m solution of iodide ions was added to a solution containing excess lead II ions.
Calculate the mass in grams of lead II iodide formed
(ii) Identify the colour of the product formed in (d) (i)
15. a) The diagram below represents part of the structure of sodium chloride crystal
The position of one of the sodium ions in the crystal is shown as;
i) On the diagram, mark the positions of the other three sodium ions
ii) The melting and boiling points of sodium chloride are 801C and 1413C respectively. Explain
why sodium chloride does not conduct electricity at 25C, but does not at temperatures
between 801C and 1413C
b) Give a reason why ammonia gas is highly soluble in water
c) The structure of ammonium ion is shown below;
Name the type of bond represented in the diagram by N H
d) Carbon exists in different crystalline forms. Some of these forms were recently discovered
in soot and are called fullerenes
i) What name is given to different crystalline forms of the same element
ii) Fullerenes dissolve in methylbenzene while the other forms of carbon do not. Given that soot is
a mixture of fullerenes and other solid forms of carbon, describe how crystals of fullerenes can
be obtained from soot
iii) The relative molecular mass of one of the fullerenes is 720. What is the molecular mass of
this fullerene
16. Calculate the volume of oxygen gas used during the burning of magnesium (O = 16, molar
gas volume = 24,000cm3 at room temperature)
17. Study the information in the table below and answer the questions that follow
i) Write the general formula of the hydrocarbons in the table
ii) Predict the relative atomic mass of the hydrocarbons with 5 carbon atoms
iii) Determine the relative atomic mass of the hydrocarbon in (ii) above and draw its
structural formula (H=1.0, C=12.0)
18. A hydrated salt has the following composition by mass. Iron 20.2 %, oxygen 23.0%,
sulphur 11.5%, water 45.3%
i) Determine the formula of the hydrated salt (Fe=56, S=32, O=16, H=11) (3 mks)
ii) 6.95g of the hydrated salt in c(i) above were dissolved in distilled water and the total
volume made to 250cm3 of solution. Calculate the concentration of the resulting salt solution
in moles per litre. (Given that the molecula mass of the salt is 278)
19. a) Galvanized iron sheets are made by dipping the sheets in molten Zinc.
i) Explain how zinc protects iron from rusting
ii) Name the process applied in galvanization of iron with zinc
20. Calculate the percentage of copper in 1.0g of the alloy
(Cu = 63.5 Mg = 24)
21. A factory uses nitric acid and ammonia gas as the only reactant for the preparation of the
fertilizer if the daily production of the fertilizer is 4800kg. Calculate the mass of ammonia
gas used daily
(N = 14.0, O= 16.0, H = 1.0)
22. Calculate the volume of sulphur (VI) oxide gas that would be required to produce 178kg of
oleum in step 3 molar gas volume at s.t.p = 22.4 litres H = 1 O = 16 S = 32
23. Using the answer in d (ii) above, determine:
i) The volume of 1M nitric acid that would react completely with one mole of copper
(Cu = 63.5)
ii) The volume of Nitrogen (IV) oxide gas produced when one mole of copper reacts
with excess 1M nitric acid at room temperature
24. A sample of biogas contains 35.2% by mass of methane. A biogas cylinder contains 5.0kg
of the gas. Calculate:
(i) Number of moles of methane in the cylinder (Molar mass of methane = 16)
(ii) Total volume of carbon (IV) oxide produced by the combustion of methane in the cylinder
(Molar gas volume = 24.0dm3 at room temperature and pressure)
25. 0.84g of aluminium were reacted completely with chlorine gas. Calculate the volume
of chlorine gas used. (Molar gas volume is 24dm3, Al = 27)
26. 3.52g of Carbon (IV) Oxide and 1.40g of water are produced when a mass of a hydrocarbon
is completely burnt in oxygen. Determine the empirical formula of the hydrocarbon;
(H = 1 , C= 12, O = 16)
27. Calculate the number of water molecules when 34.8g Na2CO3 xH2O is heated and 15.9g of
anhydrous Na2CO3 obtained (H=1, O=16, Na= 23, C = 12)
28. A weighed sample of crystallined sodium carbonate (Na2CO3nH2O) was heated in a crucible
until there was no further change in mass. The mass of the sample reduced by 14.5%. Calculate
the number of moles (n) of water of crystallization (Na = 23, O = 16, C = 12, H = 1)
29. In a reaction 20cm3 of 0.1 M Sodium Carbonate completely reacted with 13cm3 of dilute sulphuric acid. Find the molarity of the sulphuric acid used.
30. An organic compound P contains 68.9% carbon, 13.5% hydrogen and 21.6% oxygen.
The relative formula mass of p is 74. Determine its molecular formula. [C=12, H=1, 0=16]
31. Campers GAZ cylinder contains about 1.12dm3 of butane measured at 0o and 1atm. Given that
25% of heat is lost, what is the maximum volume of water at room temperature which can be
boiled to 100oC in order to make some coffee?
(Specific heat capacity of water = 4.2J g-1C-0c, density of water 1gcm-3 Molar gas volume 22.41 at s.t.p)
32. An aqueous solution containing anhydrous sodium carbonate was prepared by dissolving
19.6g of the salt in 250cm3 of distilled. Calculate the volume of 2M of magnesium chloride
solution required to precipitate all the carbonate ions in the solution.
(Na=23, C= 12; O = 16; Mg = 24; Cl =35.5)
33. 10.08g of ethanedioic acid (H2C2O4.xH2O) crystals were dissolved in water and made to
1dm3 solution. 25.0cm3 of this solution was completely neutralized by 20cm3 of 0.2M
sodium hydroxide solution.
Calculate
i) Molarity of the acid
ii)the value of x in H2C2O4xH2O acid
34. 1.6g of magnesium metal is reacted with excess hydrochloric acid. Calculate the volume
of hydrogen gas produced
(Molar gas volume at stp = 22.4dm3 Mg=24)
35. 60 litres of sulphur(IV) oxide were made to react with 40 litres of oxygen.
a) Which reactant was in excess and by how much?
b) What is the volume of the product?
36. During welding of cracked railway lines by thermite 12.0g of oxide of iron is reduced by
aluminium to 8.40g of iron. Determine the empirical formula of the oxide
(Fe= 56.0, O= 16.0)
Gas laws
1. A sample of unknown compound gas X is shown by analysis to contain Sulphur and Oxygen. The
gas requires 28.3 seconds to diffuse through a small aperture into a vacuum. An identical number
of oxygen molecules pass through the same aperture in 20seconds. Determine the molecular mass
of gas X (O= 16, S= 32)
2. (a) State Graham’s Law of diffusion
(b) Gas V takes 10 seconds to diffuse through a distance of one fifth of a meter. Another
gas W takes the same time to diffuse through a distance of 10 cm. if the relative molecular
mass of gas V is 16.0; calculate the molecular mass of W
3. (a) State Charles’ Law
(b) The volume of a sample of nitrogen gas at a temperature of 291K and 1.0 x 10^5 Pascals
was 3.5 x 10-2m3. Calculate the temperature at which the volume of the gas would be
2.8 x 10^-2 m3 at 1.0 x 10^5 pascals.
4. 60 cm3 of oxygen gas diffused through a porous partition in 50 seconds. How long would it take
60 cm3 of sulphur (IV) oxide gas to diffuse through the same partition under the sane conditions?
(S = 32.0, O = 16.0)
5. (a) State Graham’s law of diffusion
(b) 30cm3 of hydrogen chloride gas diffuses through a porous pot in 20seconds. How long
would it take 42cm3 of sulphur(IV) oxide gas to diffuse through the same pot under
the same conditions (H =1 Cl = 35.5 S = 32 O =16)
6. a) State Boyles law
b) Sketch a graph that represents Charles’ law
c) A gas occupied a volume of 250cm3 at -23ºC and 1 atmosphere. Determine its volume
at 127ºC when pressure is kept constant.
7. A factory produces Calcium Oxide from Calcium Carbonate as shown in the equation below:-
CaCO3 (s)--------> CaO (s) + CO2 (g)
(a) What volume of Carbon (IV) Oxide would be produced from 1000kg of Calcium
Carbonate at s.t.p (Ca = 40, C = 12, O = 16, Molar gas volume at s.t.p = 22.4dm3)
8. A fixed mass of gas occupies 200cm3 at a temperature of 23oC and pressure of 740mmHg.
Calculate the volume of the gas at -25 degrees C and 780mmHg pressure
9. Gas K diffuses through a porous material at a rate of 12cm3 s-1 where as S diffuses through
the same material at a rate of 7.5cm3s-1. Given that the molar mass of K is 16, calculate the
molar mass of S
10. (a) State Gay Lussac’s law
. 11. (a) What is the relationship between the rate of diffusion of a gas and its molecular mass?
(b) A sample of Carbon (IV) Oxide takes 200 seconds to diffuse across a porous plug.
How long will it take the same amount of Carbon (II) Oxide to diffuse through the
same plug?(C=12, O=16)
12. Below are structures of particles. Use it to answer questions that follow. In each case only
electrons in the outermost energy level are shown
(a) Identify the particle which is an anion
(b) Choose a pair of isotopes. Give a reason
13. The figure below shows two gases P and Q diffusing from two opposite ends 18 seconds after
the experiment
(a) Which of the gases has a lighter density?
(b) Given that the molecular mass of gas Q is 17, calculate the molecular mass of P
14. Identify the particles that facilitate the electric conductivity of the following substances
(i) Sodium metal
(ii) Sodium Chloride solution
(iii) Molten Lead Bromide
15. Gas B takes 110 seconds to diffuse through a porous pot, how long will it take for the
same amount of ammonia to diffuse under the same conditions of temperature and pressure?
(RMM of B = 34 RMM of ammonia = 17)
16. A gas occupies 5dm3 at a temperature of -27 degrees C and 1 atmosphere pressure. Calculate the
volume occupied by the gas at a pressure of 2 atmospheres and a temperature of 127 degress C
17. A fixed mass of gas occupies 200 cm3 at a temperature of 230c and a pressure of 740 mm Hg.
Calculate the volume of the gas at -25 degrees c and 790 mm Hg pressure.
18. (a) State the Graham’s law
(b) 100cm3 of Carbon (IV) oxide gas diffused through a porous partition in 30seconds.
How long would it take 150cm3 of Nitrogen (IV) oxide to diffuse through the same
partition under the same conditions? (C = 12.0, N = 14.0, O = 16.0)
gas requires 28.3 seconds to diffuse through a small aperture into a vacuum. An identical number
of oxygen molecules pass through the same aperture in 20seconds. Determine the molecular mass
of gas X (O= 16, S= 32)
2. (a) State Graham’s Law of diffusion
(b) Gas V takes 10 seconds to diffuse through a distance of one fifth of a meter. Another
gas W takes the same time to diffuse through a distance of 10 cm. if the relative molecular
mass of gas V is 16.0; calculate the molecular mass of W
3. (a) State Charles’ Law
(b) The volume of a sample of nitrogen gas at a temperature of 291K and 1.0 x 10^5 Pascals
was 3.5 x 10-2m3. Calculate the temperature at which the volume of the gas would be
2.8 x 10^-2 m3 at 1.0 x 10^5 pascals.
4. 60 cm3 of oxygen gas diffused through a porous partition in 50 seconds. How long would it take
60 cm3 of sulphur (IV) oxide gas to diffuse through the same partition under the sane conditions?
(S = 32.0, O = 16.0)
5. (a) State Graham’s law of diffusion
(b) 30cm3 of hydrogen chloride gas diffuses through a porous pot in 20seconds. How long
would it take 42cm3 of sulphur(IV) oxide gas to diffuse through the same pot under
the same conditions (H =1 Cl = 35.5 S = 32 O =16)
6. a) State Boyles law
b) Sketch a graph that represents Charles’ law
c) A gas occupied a volume of 250cm3 at -23ºC and 1 atmosphere. Determine its volume
at 127ºC when pressure is kept constant.
7. A factory produces Calcium Oxide from Calcium Carbonate as shown in the equation below:-
CaCO3 (s)--------> CaO (s) + CO2 (g)
(a) What volume of Carbon (IV) Oxide would be produced from 1000kg of Calcium
Carbonate at s.t.p (Ca = 40, C = 12, O = 16, Molar gas volume at s.t.p = 22.4dm3)
8. A fixed mass of gas occupies 200cm3 at a temperature of 23oC and pressure of 740mmHg.
Calculate the volume of the gas at -25 degrees C and 780mmHg pressure
9. Gas K diffuses through a porous material at a rate of 12cm3 s-1 where as S diffuses through
the same material at a rate of 7.5cm3s-1. Given that the molar mass of K is 16, calculate the
molar mass of S
10. (a) State Gay Lussac’s law
. 11. (a) What is the relationship between the rate of diffusion of a gas and its molecular mass?
(b) A sample of Carbon (IV) Oxide takes 200 seconds to diffuse across a porous plug.
How long will it take the same amount of Carbon (II) Oxide to diffuse through the
same plug?(C=12, O=16)
12. Below are structures of particles. Use it to answer questions that follow. In each case only
electrons in the outermost energy level are shown
(a) Identify the particle which is an anion
(b) Choose a pair of isotopes. Give a reason
13. The figure below shows two gases P and Q diffusing from two opposite ends 18 seconds after
the experiment
(a) Which of the gases has a lighter density?
(b) Given that the molecular mass of gas Q is 17, calculate the molecular mass of P
14. Identify the particles that facilitate the electric conductivity of the following substances
(i) Sodium metal
(ii) Sodium Chloride solution
(iii) Molten Lead Bromide
15. Gas B takes 110 seconds to diffuse through a porous pot, how long will it take for the
same amount of ammonia to diffuse under the same conditions of temperature and pressure?
(RMM of B = 34 RMM of ammonia = 17)
16. A gas occupies 5dm3 at a temperature of -27 degrees C and 1 atmosphere pressure. Calculate the
volume occupied by the gas at a pressure of 2 atmospheres and a temperature of 127 degress C
17. A fixed mass of gas occupies 200 cm3 at a temperature of 230c and a pressure of 740 mm Hg.
Calculate the volume of the gas at -25 degrees c and 790 mm Hg pressure.
18. (a) State the Graham’s law
(b) 100cm3 of Carbon (IV) oxide gas diffused through a porous partition in 30seconds.
How long would it take 150cm3 of Nitrogen (IV) oxide to diffuse through the same
partition under the same conditions? (C = 12.0, N = 14.0, O = 16.0)
Sunday, 24 November 2019
Effect of an electric current on substances
1. The set-up was used to electrolyse Lead (II) bromide. Study it and answer the questions
that follow;
(a) Write an ionic equation for the reaction that occurred at the cathode
(b) State and explain what happened at the anode
2. When an electric current was passed through two molten substances E and F in separate
voltammeters. The observations recorded below were made:-
Complete the table above
3. (a) Differentiate the following terms :-
Electrolyte and non-electrolyte
(b) The diagram below is a set-up used to investigate the conductivity of electric current
by some aqueous solution. Study it and answer the questions that follow;
(i) State the observation made on the bulb when each of the following solution were put
onto the beaker
(a) Sugar solution
(b) (i) Salt solution
(ii) Classify the substance in (i) above as either electrolyte or non-electrolyte
(b) If in the above set-up of apparatus, the substance to be tested is Lead II Bromide,
what modification should be included in the set-up?
(c) Write an Ionic equation at the electrodes and state the observation:-
Anode
4. (a) The diagram below shows the set up used to investigate the effect of an electric current
on molten lead (II) bromide
i. Explain what happens to the lead II bromide during electrolysis
ii. Why is it important to carry out the experiment in a fume chamber?
5. (I) Define the following terms:
(a) Crystallization
(b) (i) Salting out as used in soap making
(ii) Starting with barium carbonate solid, dilute sulphuric acid and dilute nitric acid,
describe how you would prepare dry barium sulphate solid
(iii) Study the scheme below and answer the questions which follow:
(a) Identify ;
(i) The cation present in solid S
(ii) The anion in solid S
(b) Write an equation to show how solid S is heated in process T
(iv) Copper II chloride solution dissolves in excess ammonia solution to form a deep blue
solution. Give the ion responsible for the deep blue solution
(v) A solution of hydrogen chloride is an electrolyte but a solution of hydrogen chloride in
methylbenzene in a non-electrolyte. Explain
6. (i) State Faraday’s first law of electrolysis
(ii) The diagram below shows a set-up used for the electrolysis of molten Lead bromide:-
State the observations that would be made at the anode and cathode as the electrolysis progressed
7. (a) (i) Describe how you would prepare pure crystals of lead II nitrate in the laboratory from
lead II oxide
(ii) Write an equation for the reaction that takes place in (a)(i) above
(b) (i) State what happens when lead II nitrate is strongly heated
(ii) Write an equation for the reaction in b(i) above
(c) (i) State what is observed when ammonia solution is gradually added to a solution of lead II nitrate until the alkali is in excess
(ii) Write an ionic equation for the reaction that takes place in (i) above
8. The diagram show an experiment for investigating electrical conduction in lead (II) fluoride.
Study it and answer the questions that follow:
(a) On the diagram
(i) Label the anode and the cathode
(ii) Show the direction of movement of electrons
(iii) Complete the diagram by indicating the condition that is missing but must be present for
electrical conduction to take place.
(b) Why is it necessary to leave a gap between the cork and the boiling tube?
(c) State the observations that are expected at the electrodes during electrical conduction and
at the experiment
(d) Write equations for the reactions that take place at the electrodes
(e) Why should this experiment be carried out in a fume chamber?
II. The table below shows the electrical conductivity of substance A, B and C
(a) Which one of the substance is likely to be plastic?
(b) Explain why the substance you have given in (a) above behaves in the way it does
(c) Which of the substances is likely to be sodium chloride? Explain
(d) Give the type of structure and bonding that is present in substance A
9. Study the diagram below and use it to answer the questions that follow:-
(a) Identify electrodes A and B
(b) Name the product formed at the anode
(c) Write the electrode half equation of reaction at electrode A
10. Explain the differences in electrical conductivity between melted sodium chloride and
liquid mercury
11. Below is part of a flow diagram for the contact process:
(a) Name :
I. Liquid Y ……………………………………………………….
II. Liquid N………………………………………………………….
(b) Write the equation for the reaction taking place in;
I. Chamber Q
II. Chamber R
12. In an experiment to investigate the conductivity of substances, a student used the set-up shown
below.
The student noted that the bulb did not light.
a) What had been omitted in the set up.
b) Explain why the bulb lights when the omission is corrected.
that follow;
(a) Write an ionic equation for the reaction that occurred at the cathode
(b) State and explain what happened at the anode
2. When an electric current was passed through two molten substances E and F in separate
voltammeters. The observations recorded below were made:-
Complete the table above
3. (a) Differentiate the following terms :-
Electrolyte and non-electrolyte
(b) The diagram below is a set-up used to investigate the conductivity of electric current
by some aqueous solution. Study it and answer the questions that follow;
(i) State the observation made on the bulb when each of the following solution were put
onto the beaker
(a) Sugar solution
(b) (i) Salt solution
(ii) Classify the substance in (i) above as either electrolyte or non-electrolyte
(b) If in the above set-up of apparatus, the substance to be tested is Lead II Bromide,
what modification should be included in the set-up?
(c) Write an Ionic equation at the electrodes and state the observation:-
Anode
4. (a) The diagram below shows the set up used to investigate the effect of an electric current
on molten lead (II) bromide
i. Explain what happens to the lead II bromide during electrolysis
ii. Why is it important to carry out the experiment in a fume chamber?
5. (I) Define the following terms:
(a) Crystallization
(b) (i) Salting out as used in soap making
(ii) Starting with barium carbonate solid, dilute sulphuric acid and dilute nitric acid,
describe how you would prepare dry barium sulphate solid
(iii) Study the scheme below and answer the questions which follow:
(a) Identify ;
(i) The cation present in solid S
(ii) The anion in solid S
(b) Write an equation to show how solid S is heated in process T
(iv) Copper II chloride solution dissolves in excess ammonia solution to form a deep blue
solution. Give the ion responsible for the deep blue solution
(v) A solution of hydrogen chloride is an electrolyte but a solution of hydrogen chloride in
methylbenzene in a non-electrolyte. Explain
6. (i) State Faraday’s first law of electrolysis
(ii) The diagram below shows a set-up used for the electrolysis of molten Lead bromide:-
State the observations that would be made at the anode and cathode as the electrolysis progressed
7. (a) (i) Describe how you would prepare pure crystals of lead II nitrate in the laboratory from
lead II oxide
(ii) Write an equation for the reaction that takes place in (a)(i) above
(b) (i) State what happens when lead II nitrate is strongly heated
(ii) Write an equation for the reaction in b(i) above
(c) (i) State what is observed when ammonia solution is gradually added to a solution of lead II nitrate until the alkali is in excess
(ii) Write an ionic equation for the reaction that takes place in (i) above
8. The diagram show an experiment for investigating electrical conduction in lead (II) fluoride.
Study it and answer the questions that follow:
(a) On the diagram
(i) Label the anode and the cathode
(ii) Show the direction of movement of electrons
(iii) Complete the diagram by indicating the condition that is missing but must be present for
electrical conduction to take place.
(b) Why is it necessary to leave a gap between the cork and the boiling tube?
(c) State the observations that are expected at the electrodes during electrical conduction and
at the experiment
(d) Write equations for the reactions that take place at the electrodes
(e) Why should this experiment be carried out in a fume chamber?
II. The table below shows the electrical conductivity of substance A, B and C
(a) Which one of the substance is likely to be plastic?
(b) Explain why the substance you have given in (a) above behaves in the way it does
(c) Which of the substances is likely to be sodium chloride? Explain
(d) Give the type of structure and bonding that is present in substance A
9. Study the diagram below and use it to answer the questions that follow:-
(a) Identify electrodes A and B
(b) Name the product formed at the anode
(c) Write the electrode half equation of reaction at electrode A
10. Explain the differences in electrical conductivity between melted sodium chloride and
liquid mercury
11. Below is part of a flow diagram for the contact process:
(a) Name :
I. Liquid Y ……………………………………………………….
II. Liquid N………………………………………………………….
(b) Write the equation for the reaction taking place in;
I. Chamber Q
II. Chamber R
12. In an experiment to investigate the conductivity of substances, a student used the set-up shown
below.
The student noted that the bulb did not light.
a) What had been omitted in the set up.
b) Explain why the bulb lights when the omission is corrected.
Subscribe to:
Comments (Atom)