(b) Both graphite and diamond are allotropes of element Carbon. Graphite conducts electricity whereas diamond does not. Explain
2. Below is a simplified scheme of solvay process. Study it and answer the questions that follow:
b) Write an equation for the process III.
c) Give one use of sodium carbonate.
3. A burning magnesium continues to burn inside a gas jar full of carbon (IV) oxide. Explain.
4. The diagram below shows a jiko when in use
(a) Identify the gas formed at region H
(b) State and explain the observation made at region G
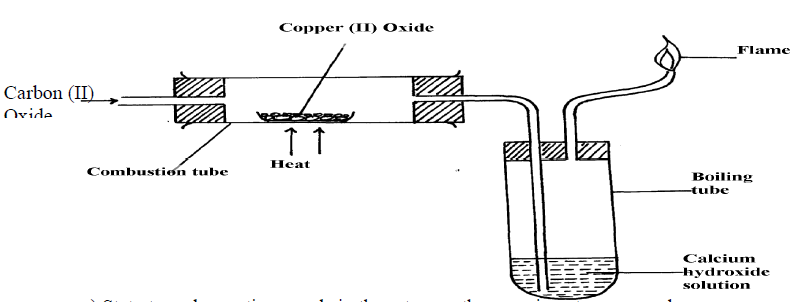
5. Study the diagram below and use it to answer the questions that follow.
(a) State the observation made in the combustion tube.
(b) Write an equation for the reaction that took place in the combustion tube
(c) Give one use of P
6. (a) Identify two substance that are reacted to regenerate ammonia gas in the solvary process
(b) Write down a balanced chemical equation for the reaction above
7. When the oxide of element H was heated with powdered Carbon, the mixture glowed and Carbon (IV) oxide was formed. When the experiment was repeated using the oxide of element J, there was no apparent reaction
(a) Suggest one method that can be used to extract element J from its oxide
(b) Arrange the elements H, J and Carbon in order of their decreasing reactivity
8. (i) Diamond and silicon (IV) Oxide have a certain similarity in terms of structure and bonding. State it
(ii) State one use of diamond
9. (a) What is allotropy?
(b) Diamond and graphite are allotropes of Carbon. In terms of structure and bonding
explain why graphite conducts electricity but not diamond
10. The diagram below shows a charcoal stove with different regions
(b) How would one avoid the production of the product at B? Give a reason for your answer
11. Study the diagram below and answer the questions that follow:
(b) Write an equation for the reaction that takes place in the combustion tube
12. Diamond and graphite are allotropes of carbon:-
(a) What is meant by allotropes?
(b) How do they differ in their structure and bonding
13. Study the experimental set-up below:
b) By use of a chemical equation, explain the changes that occurred in the boiling tube
c) Why was it necessary to burn the excess gas?
14. The diagram below shows the heating curve of a pure substance. Study it and answer the
questions that follow:
(d) The substance under test is definitely not water; Give a reason for this
(e) What would happen to the melting point of this substance if it were contaminated with sodium chloride?
(f) What happens to the temperature between points B and C?
15. Study the set-up below and answer the questions that follow:
(ii) State the effect of releasing gas X to the environment
(b) Write down equations for the reactions taking place in;
(i) Tube I
(ii) Tube II
(iii) Flask
(c) State the observation made in tube III
(d) Write down an equation for the reaction which could be used to generate Carbon
(IV) Oxide for the above set up
(e) Name the reagents used to generate gas x in the laboratory
(f) Complete the diagram above to show how excess gas x can be collected
16. The figure below shows the stages in the manufacture of sodium carbonate. Study the diagram
below and use it to answer the questions that follow.
(ii) Which substances are recycled in this process?
(iii) Identify the chambers in which the recycled substances are regenerated.
(iv) Name the substances U and V.
b) Give an equation for the reaction which occurs:
(i) In the reaction chamber 1
(ii) When solid V is heated.
(iii) In the reaction chamber 3.
c) State one commercial use for
(i) Sodium carbonate.
17. The set-up below was used to prepare dry carbon (II) Oxide gas. use it to answer the questions
below it:
(ii) The student produced carbon (IV) oxide gas from the reaction between Lead (II) Carbonate
and dilute hydrochloric acid. The gas was produced for a short time and the reaction came
to a stop. Explain
(iii) Write the equation for the reactions taking place in the combustion tube and the conical
flask:
Combustion tube:…………………………………………………………………..
Conical flask ……………………………………………………………………..
(iv) State one use of carbon (IV) Oxide gas apart from fire extinguisher
(v) Give two properties that make carbon (IV) Oxide to be used as fire extinguisher
(iv) State one use of carbon (IV) Oxide gas apart from fire extinguisher
(v) Give two properties that make carbon (IV) Oxide to be used as fire extinguisher
(b)
Which property of carbon (II) Oxide is demonstrated by the above equation?
(c) Aluminium carbonate does not exist. Give a reason
(d) Ammonium carbonate decomposes when heated. Write a chemical equation to
represent this decomposition
18. State and explain the observation made when a piece of charcoal is dropped in a jar containing concentrated nitric (V) acid
19. When Carbon (IV) oxide is passed through lime water, a white precipitate is formed but when excess Carbon (IV) Oxide is passed, the white precipitate disappears;
(a) Explain why the white precipitate disappears
(b) Give an equation for the reaction that takes place in (a) above
20. The set-up below was used to prepare a carbon (II) oxide gas.
(b) Complete the diagram to show how the gas can be collected
(c)Write the equation for the reaction



















Kindly show answers
ReplyDeletePlz
DeleteYes
DeleteThese materials are sometimes given as assignment to learners by their instructors and consequently we can't show the answers here but you can engage me through WhatsApp on +254724401612.
ReplyDeleteI will be glad if you a comment regarding the usefulness of these materials.
Really nice and interesting post. I was looking for this kind of information and enjoyed reading this one. Electrode Carbon Paste
ReplyDeleteThis is very good revision material.Keep up the good work
ReplyDeleteI'm happy to see the considerable subtle element here!. I'm no expert, but I believe you just made an excellent point. You certainly fully understand what youre speaking about, and
ReplyDeleteI can truly get behind that.best fire extinguisher for home
That is truly decent to listen. much obliged to you for the upgrade
ReplyDeleteand good fortunes. https://1asiaqq.net
pliz can you show the answers...or is it a must we engage in a convo on WhatsApp some of us aint comfortable chatting in the dm
ReplyDeletepliz can you show the answers...or is it a must we engage in a convo on WhatsApp some of us aint comfortable chatting in the dm
ReplyDeleteDid you get the answers
Delete